Brain disorders come in a kaleidoscope of forms, from involuntary movements to intrusive thought. But beneath this variety lies a common thread: the presence of dysfunctional brain connections. To target these dysfunctions precisely and effectively, we need to establish an unequivocal link between brain circuit and symptom. Yet, mappings resulting from traditional methods like functional brain imaging have often raised new questions with significant implications for clinical translation, namely whether the established relationship may be of causal, correlational, or incidental nature.
Enter deep brain stimulation (DBS), a promising tool in our quest to understand and treat these complex conditions. By delivering weak electrical pulses through small electrodes implanted within deep brain structures, DBS not only eases symptoms but also offers a window into the network architecture of the brain’s dysfunction. Here’s why: First, DBS targets specific brain areas with remarkable precision. Second, it often leads to significant symptom improvements. Third, it creates an ‘informational’ lesion, downregulating aberrant information processing in dysfunctional circuits.
Combined with advanced high-resolution connectomics, these properties may confer DBS a unique potential for uncovering which dysfunctional circuits need to be modulated for maximized symptom relief. Think of DBS as a prism, splitting light into a rainbow of colors. Similarly, DBS may be able to segregate the human connectome into different symptom networks relevant for each disorder.
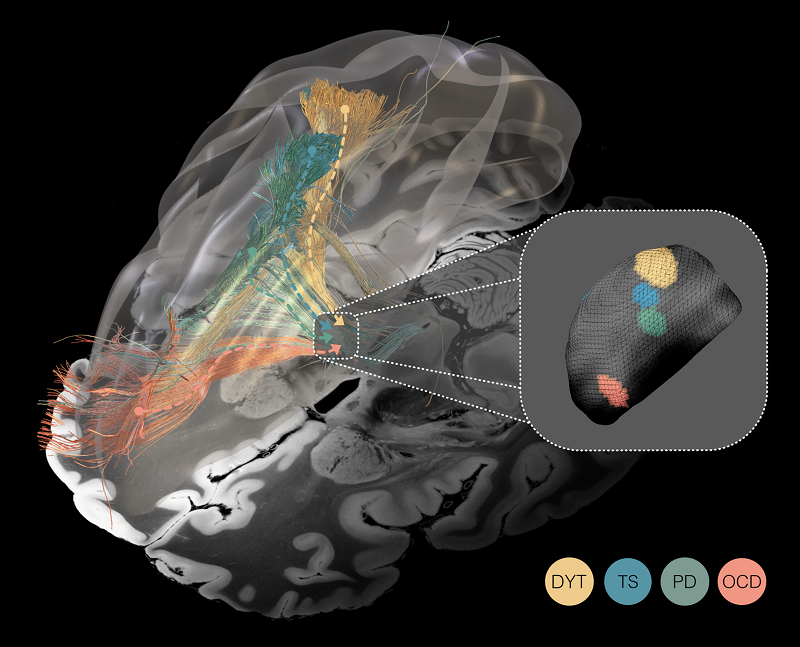
Unveiling the spectrum of the ‘dysfunctome’ through the prism of deep brain stimulation. Deep brain stimulation functions akin to a prism fragmenting the light, in that it segregates the connectome into dysfunctional circuits whose stimulation was associated with symptom improvement in Parkinson’s disease (PD), dystonia (DYT), Tourette’s syndrome (TS), and obsessive-compulsive disorder (OCD). Mappings are superimposed onto a 100 μm ex-vivo brain template for anatomical reference (Edlow et al. 2019). Inset: Optimal stimulation sites within the same small subcortical nucleus — the subthalamic nucleus — for all four disorders.
With this idea in mind, we set out to explore whether DBS could effectively uncover dysfunctional brain circuits linked to Parkinson’s disease, dystonia, obsessive-compulsive disorder, and Tourette’s syndrome. Tackling this question was enabled by invaluable (retrospective) data from 261 patients who had a total of 534 DBS electrodes implanted, graciously shared by ten DBS centers spanning seven countries. Electrode placement within the same, spatially circumscribed target area — the subthalamic nucleus — for all four disorders allowed for comparative analysis of dysfunctional circuits using precise electrode localization and computer simulations.
Identified circuits segregated the frontal cortex into distinct territories, ranging from occipital to frontal: Targeting dysfunctional connectivity between deep brain structures and the sensorimotor cortex in dystonia, the primary motor cortex in Tourette‘s syndrome, the supplementary motor cortex in Parkinson‘s disease, and aspects of the cingulate cortex in obsessive-compulsive disorder emerged as crucial for therapeutic success. However, partial overlap in these circuits suggests that dysfunctions in these disorders may not be entirely isolated from one another. Focally, a similar — but miniaturized — organizational topography of dysfunction mappings was mirrored within the subthalamic target structure.
Now, why does this all matter for patient treatment? First, this spatial scaling effect of dysfunction mappings — from cortex to core — may explain why heterogeneous disorders can be treated via small electrodes placed within the same subcortical network hub. It also underlines the importance of meticulous targeting where millimeter differences matter. Excitingly, collaborations with DBS centers in Boston, Würzburg and São Paulo culminated in first clinical validations of identified mappings as therapeutic targets. Circuit-guided fine-tuning of electrode placement and stimulation parameters in three prospective patients revealed their potential to translate into real-world clinical applications. Before widespread clinical implementation, however, systematic prospective clinical trials will be essential to further validate our findings, given their reliance on retrospective data and limited sample sizes in some of the investigated disorders, particularly in the emerging application of DBS to the subthalamic nucleus for Tourette’s syndrome.
From a bigger perspective, these findings contribute to the evolving landscape of causal brain mapping, united in the pursuit of a shared mission: to chart a comprehensive ‘roadmap’ of dysfunctional brain connections and their intricate relationship with various disorders in hopes of facilitating more tailored brain circuit therapeutics. As we forge ahead, increasingly granular (somatotopic or symptom) mappings may pave the way to personalized treatment by allowing to flexibly address the unique symptom profiles of individual patients. With advancing data collection and spatial imaging accuracy, this joint effort may, one day, result in the delineation of what we coin as the human ‘dysfunctome’ — a concept akin to that of the connectome or genome. In the ever-evolving world of brain circuit therapeutics, one thing is certain: the future of the ‘dysfunctome’ holds a vibrant spectrum of possibilities!
Barbara Hollunder is pursuing a PhD as a fellow of the Einstein Center for Neurosciences Berlin and the Berlin School of Mind and Brain. She is a member of the Network Stimulation Laboratory, which is embedded within the Movement Disorders and Neuromodulation Unit at Charité Berlin and the Center for Brain Circuit Therpeutics at Brigham and Women’s Hospital Boston. Her scientific work is dedicated to investigating network effects of therapeutic neuromodulation using neuroimaging-based connectomics.
This study was also the cover story of the March 2024 edition of Nature Neuroscience. You can also learn more about this publication here.
Learn more in the original research article:
Mapping dysfunctional circuits in the frontal cortex using deep brain stimulation
Hollunder B, Ostrem JL, Sahin IA, Rajamani N, Oxenford S, Butenko K, Neudorfer C, Reinhardt P, Zvarova P, Polosan M, Akram H, Vissani M, Zhang C, Sun B, Navratil P, Reich MM, Volkmann J, Yeh FC, Baldermann JC, Dembek TA, Visser-Vandewalle V, Alho EJL, Franceschini PR, Nanda P, Finke C, Kühn AA, Dougherty DD, Richardson RM, Bergman H, DeLong MR, Mazzoni A, Romito LM, Tyagi H, Zrinzo L, Joyce EM, Chabardes S, Starr PA, Li N, Horn A. . Nat Neurosci. 2024 Mar;27(3):573-586. Epub 2024 Feb 22.
News Types: Community Stories
