By Nurunisa Akyuz
Hearing starts as sound waves are funneled into the ear canal and transmitted through the middle ear into the fluid-filled cochlea. Within the cochlea, dedicated sensory receptor cells, called ‘hair-cells’ are responsible for the conversion of the sound-induced mechanical vibrations into electrical signals. These hair-cells are named after the bundles of hair-like protrusions on their apical surfaces. When the cochlea is excited by sound, vibrations of the inner ear fluids deflect the hair-bundles. This leads to ion channel activity, generating receptor currents. This process, also known as auditory mechanotransduction (MT) has been studied extensively with hair cell electrophysiology. But despite the importance of the process, the molecular identity and the mechanism of the MT channels had eluded scientists for decades (Corey and Holt 2016). Our goal was to solve this mystery, figuring out the molecules and mechanisms responsible for turning mechanical vibrations in the inner ear into electrical signals that our brains can process as sounds.
Many ion channels assemble as macromolecular protein complexes consisting of a central pore surrounded by auxiliary proteins. Our recent work established that the TMC1 (Transmembrane Channel-Like 1) protein, which was discovered in 2002 (Kurima, et al. 2002), is the pore-forming protein that allows ions to enter the hair cell (Pan, et al. 2018). Surprisingly, our experiments also revealed that TMC1 proteins assemble as dimers, suggesting that they may contain two distinct ion pores (Pan, et al. 2018), rather than a single central one. We generated a three-dimensional architectural model for TMC1 based on the available X-ray and cryo-electron microscopy structures of similar proteins, which allowed us to visualize an ion pore within each subunit of the dimer. We don’t know yet if the opening of each pore is independent from the opening of the other.
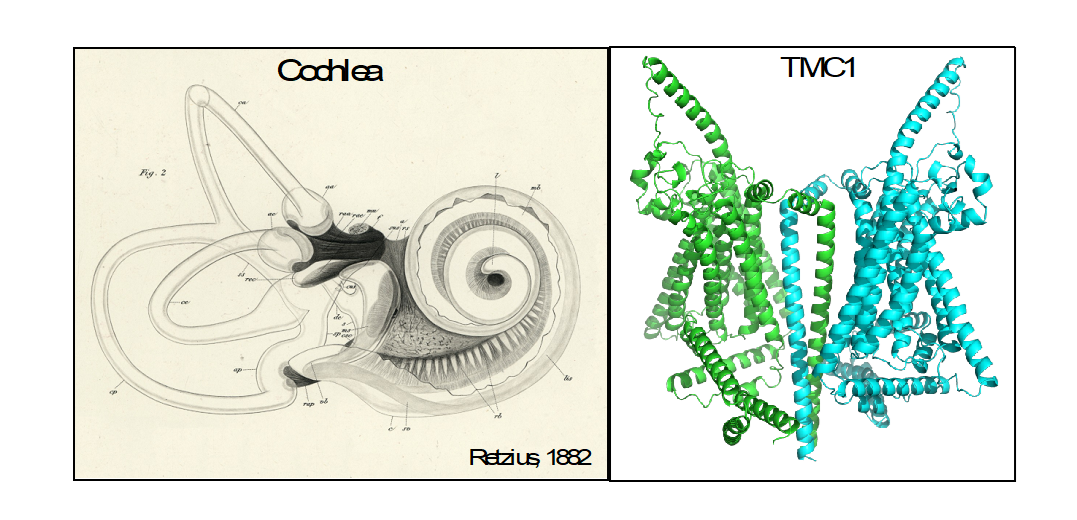
Zooming in a thousand-fold: After more than a hundred years since the detailed descriptions of the inner ear structures by the Swedish physician Gustaf Retzius (on the left), we are now working on the mechanisms of the individual molecules (on the right). The TMC1 proteins, modelled as dimers (each subunit shown with a different color), are responsible for turning sound vibrations into sound into electrical signaling – the language of our brain.
In our new work, we use the AlphaFold artificial-intelligence (AI) system (Jumper, et al. 2021) to predict the structural changes in TMC1 that are associated with channel opening (Akyuz, et al. 2022). The predicted structures suggest movements of pore-lining helices relative to each other, allowing each pore to widen or narrow, controlling ion flow. We designed a set of mutations within these helices in TMC1. To assay the effects of the mutations on the opening of the channels, we turned to native hair cells. We started recording currents from cochlear hair-cells of mice expressing virally transduced TMC1-mutants and lacking the native TMC1 and 2 genes, as we did previously (Pan, et al. 2018). We found that mutations of residues within these helices were indeed changing the extent of hair-bundle deflection that is required to open half of the channels. Furthermore, mutations of some of the residues also showed an apparent change in how efficiently the mechanical stimulus is coupled to channel opening.
We still don’t know how the sound-induced mechanical stimulus is transmitted onto TMC1. How is the hair-bundle movement coupled to channel opening? Is TMC1 an intrinsically mechanosensitive channel? Or do the auxiliary proteins in the channel complex sense the mechanical stimulus and open the TMC1 pore? Our findings thus far implicate TMC1 as part of the mechanical sensor itself. But most of the mechanosensitive channels we know about are modulated by lipid tension, suggesting a role for lipids here as well, and leaving a lot of unanswered questions for us to continue to explore.
Nurunisa Akyuz is a Research Associate in the Department of Neurobiology. She is working in David P. Corey’s laboratory.
Learn more in the original research article:
Mechanical gating of the auditory transduction channel TMC1 involves the fourth and sixth transmembrane helices.
Akyuz N, Karavitaki KD, Pan B, Tamvakologos PI, Brock KP, Li Y, Marks DS, Corey DP. Sci Adv. 2022 Jul 15;8(28)
News Types: Community Stories
