Alzheimer’s disease (AD) is a devastating neurodegenerative disorder that affects millions of people worldwide. The vast majority of patients with AD have “sporadic,” late-onset AD (LOAD). Clinicians and pathologists have long recognized that LOAD is heterogeneous as some patients have a high pathological burden and dementia, while some patients have an equally high pathological burden, but have normal cognition. This heterogeneity may translate into the poor outcomes of drugs that have gone through clinical trials for AD. With no high-efficacy therapies for the treatment of Alzheimer’s dementia, there is a need for better understanding of the underlying pathways that lead to cognitive decline. However, mouse models do not reflect the genetic heterogeneity of the aged human brain. Further, there may not be a “one size fits all” treatment that is disease modifying in every Alzheimer’s patient. Here, we sought out to generate a new resource to probe the heterogeneity of LOAD and to gain insight into pathways leading to cognitive decline.
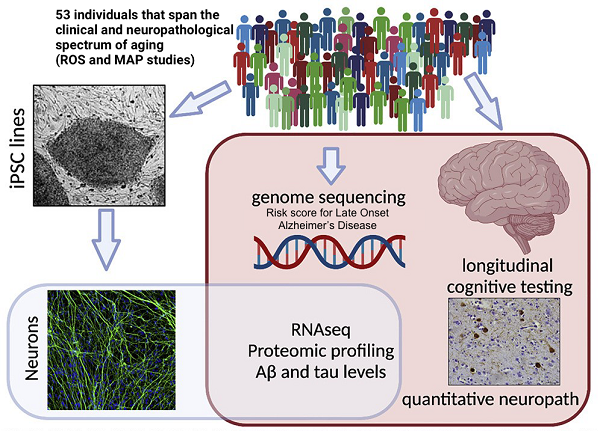
We generated iPSC lines from 53 deeply phenotyped aged humans and used them to examine the degree of congruence between iPSC derived neurons and the brain of the same person. We utilized this resource to study the association of cognitive decline and protein levels related to Alzheimer’s disease in vitro
We have generated induced pluripotent stem cell (iPSC) lines from 53 deceased individuals in the Religious Order Study (ROS) or Rush Memory and Aging Project (MAP) that span the clinical and neuropathological spectrum of aging. We differentiated these iPSC lines into cortical neurons and have profiled the cells in an unbiased manner using RNA sequencing and mass spectrometry. In addition, we have quantified several types of aβ and tau, the two proteins that accumulate in the brains of Alzheimer’s patients. We identified specific proteins and pathways in neuronal cultures that are associated with AD polygenic risk score, neuropathological burden, and/or cognitive trajectory in the donors and validated these findings through analyses of brain tissue from the same individuals. As one example of the utility of this resource for probing events relevant to AD, we identified and then experimentally validated a mechanistic link between aβ peptides and tau via protein phosphatase 1, a complex repeatedly associated with AD-related phenotypes in this study. We believe this collection of iPSC lines provides a readily manipulatable system for future studies that interrogate genetic pathways underlying LOAD and the aging human brain. To learn more about the work of the Young-Pearse lab, please visit: https://youngpearselab.bwh.harvard.edu/ or follow the lab on Facebook: https://www.facebook.com/YoungPearseLab/.
Valentina Lagomarsino is a graduate student in the lab of Dr. Meenaskshi Rao at Boston Children’s Hospital. She completed this study while a research assistant In the lab of Dr. Tracy Young-Pearse, along with joint first author Richard V. Pearse II.
Learn more in original research article:
Stem cell-derived neurons reflect features of protein networks, neuropathology, and cognitive outcome of their aged human donors
Valentina N. Lagomarsino*, Richard V. Pearse II*, Lei Liu, Yi-Chen Hsieh, Marty A. Fernandez, Elizabeth A. Vinton, Daniel Paull, Daniel Felsky, Shinya Tasaki, Chris Gaiteri, Badri Vardarajan, Hyo Lee, Christina R. Muratore, Courtney R. Benoit, Vicky Chou, Seeley B. Fancher, Amy He, Julie P. Merchant, Duc M. Duong, Hector Martinez, Monica Zhou, Fatmata Bah, Maria A. Vicent, Jonathan M.S. Stricker, Jishu Xu, Eric B. Dammer, Allan I. Levey, Lori B. Chibnik, Vilas Menon, Nicholas T. Seyfried, Philip L. De Jager, Scott Noggle, Dennis J. Selkoe, David A. Bennett, and Tracy L. Young-Pearse. Neuron. 2021 Aug 26:S0896-6273(21)00578-X.
News Types: Community Stories
