By Jeffrey Schweitzer
Parkinson’s disease is among the most prevalent movement disorders, affecting 1% of the population over age 50—a number expected to double or triple over the next few decades as the population ages. While conventional medical therapies remain the mainstay of care, it has long been recognized that they have significant limitations due to decreasing efficacy and increasing side effects over time, worsening with age.
A major hallmark of Parkinson’s disease (PD) is selective degeneration of dopamine neurons in the midbrain. Thus, for decades, cell replacement therapy has been explored as a strategy to reverse disease progression. In particular, transplantation of dopamine cells from human fetal brain tissues, a topic of active research since the 1980s, demonstrated that grafted cells can re-innervate the brain and improve symptoms in some patients. However, results in clinical trials using these fetal tissues were mixed, and the use of this tissue source has obvious practical and ethical problems, prompting a search for a more optimal cell source.
Over the last two decades, progress in the technology of creating stem cells from adult tissues and then guiding them in vitro to become replacement dopamine neurons has heightened interest in using this method to produce the desired cells. Refinements in the safety and efficiency of the process, such as described in a recent publication from our laboratory (Song et al, J Clin Invest 2020), have now made it possible to approach clinical trials in humans. In particular, the use of autologous cells—taken from the individual patient, rather than using stock cell lines to manufacture the dopamine cells—may make it possible to do such transplants without the need for lifelong immunosuppression and other complicating factors.
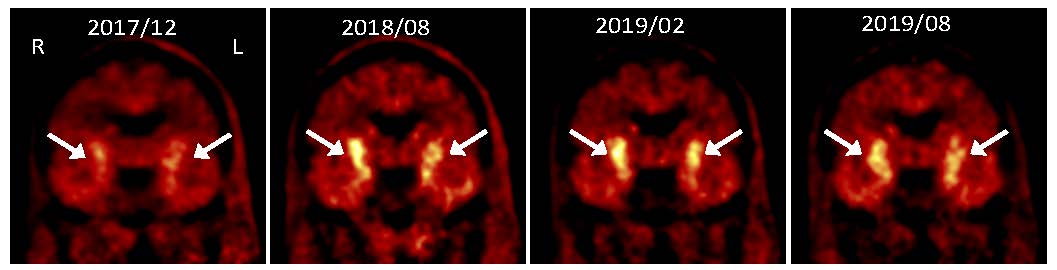
PET images showing progressive increase in uptake of dopamine precursor 18F-DOPA following grafting of dopaminergic neurons made from autologous induced pluripotent stem cells
We have reported a pilot study (Schweitzer et al, NEJM 2020), in which a patient with advanced Parkinson’s disease underwent such personalized cell therapy under a single patient approval from the FDA. The patient was then followed for over two years using clinical evaluation and imaging studies. The results show that the procedure appears to be safe, that the dopamine cells display evidence of function, and that there was improvement in symptoms. Results from this study also point out important areas of attention for planned future clinical trials. Importantly, the cells did not provoke an immune response, demonstrating the additional advantages of the personalized cell therapy approach for Parkinson’s disease and potentially for other degenerative diseases of the nervous system.
Jeffrey Schweitzer MD, PhD is an Assistant Professor of Neurosurgery at Massachusetts General Hospital.
Learn more in the original research article:
Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. Schweitzer, J. S., Song, B., Herrington, T. M., Park, T. Y., Lee, N., Ko, S., Jeon, J., Cha, Y., Kim, K., Li, Q., Henchcliffe, C., Kaplitt, M., Neff, C., Rapalino, O., Seo, H., Lee, I. H., Kim, J., Kim, T., Petsko, G. A., Ritz, J., … Kim, K. S. (2020). The New England Journal of Medicine, 382(20), 1926–1932. https://doi.org/10.1056/NEJMoa1915872
News Types: Community Stories
