by Jin Cui, Frederick Shipley, Neil Dani, Mark Andermann, and Maria Lehtinen
Cerebrospinal fluid (CSF) is an essential part of the central nervous system (CNS), guiding the brain’s development, ensuring that its nutritional needs are met, and protecting it from mechanical injury during movement. The choroid plexus (ChP), floating deep in each of the brain’s ventricles, is a primary source of this critical fluid and forms the blood-CSF barrier. The ChP also orchestrates neuroimmune responses. How the ChP performs these activities has been unclear, in part because its anatomical location has made direct observation impossible with available methodology. Here we highlight two new innovative approaches developed in a collaboration between the Lehtinen and Andermann labs that capture, for the first time, the secret life of the choroid plexus in vivo. The studies reveal the secretory and calcium dynamics of ChP epithelial cells, which are responsible for secreting CSF and its principal components, as well as movements and functions of macrophages, which represent the largest class of immune cells residing in the ChP.
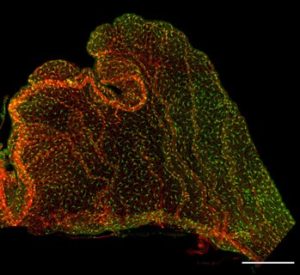
An adult choroid plexus explant from the Cx3cr1-GFP transgenic mouse, stained wtih PECAM to label the vasculature.
In the first study (Shipley, Dani, et al., Neuron 2020), we present two platforms for exploring ChP function in adult mice on a cellular level. In the first platform, we microdissected the ChP from the lateral ventricle and stabilized it either for immunostaining or for live imaging of epithelial cells. Using the genetically encoded calcium indicator GCaMP6f, we observed calcium activity and secretory events. Informed by gene expression studies that revealed the expression of serotonin receptors by ChP epithelial cells, we tested and found that serotonergic agonists increase calcium activity and frequency of secretory events. Calcium activity of cells can also be visualized deep inside the adult mouse brain using multi-photon imaging. Therefore, in the second platform, we surgically implanted an imaging window – a “skylight” – into adult mice.
This skylight allowed us to directly see and image the choroid plexus in its natural state — its physiological niche inside the lateral ventricle of awake mice. In addition to spontaneous subcellular calcium events, we found that a peripherally delivered serotonin agonist can trigger large and rapid release of cellular contents from the ChP epithelium directly into the CSF. In a parallel approach, we applied our in vivo imaging platform to ChP immune cells, and uncovered diverse subpopulations, differentiated by their location (on the CSF-facing side or on the blood-facing side of the epithelium) and function (e.g., motility, mobility, surveillance, housekeeping, and rapid response to acute peripheral or focal injuries).
In the second study (Cui et al., Developmental Cell 2020), we focused on the embryonic brain. Immune system disturbances resulting from maternal inflammation during pregnancy are known to increase the susceptibility of offspring to neurodevelopmental disorders. However, how the inflammatory signals make their way from the placenta to the developing fetal brain has remained a mystery.
We used a maternal immune activation (MIA) model to mimic viral infection in pregnant mice, and tested whether the embryonic ChP participates in inflammatory responses in the fetal brain. The elicited immune response recapitulated the dysregulation of cerebral cortical development described by others, which mirrors aspects of autism spectrum disorders (ASDs) in offspring (Choi et al., 2016 Science; Estes and McAllister, 2016 Science; Malkova et al., 2012 Brain. Behav. Immun.). Remarkably, we found that MIA triggered the accumulation of phagocytic macrophages at the embryonic ChP and a pro-inflammatory CSF signature. Among the upregulated cytokines, the chemoattractant CCL2 was the most robustly increased in both the CSF and ChP.
To better understand this process, we adapted the imaging toolkit from Shipley, Dani et al., to track embryonic ChP macrophage behaviors in real time. Consistent with a role in immune surveillance, embryonic ChP macrophages are constantly moving, as if they are surveying their immediate environment. Interestingly, these macrophages have shorter and fewer processes in embryos than in adults, which indicates they are still developing at this early age. Our quantitative analyses revealed that cell motility and mobility were highly correlated and showed a trend toward higher cell motility and mobility following CSF-CCL2 augmentation. This live imaging platform should allow detailed cellular imaging studies not only of macrophages but also of other cell types in ChP and nearby tissues in live embryos.
We also found that augmenting CSF-CCL2 was sufficient to disrupt the ChP epithelial cell barrier and drive ChP immune cell recruitment, proliferation, and activation. This coordinated response culminated in ChP macrophages relocating to the ChP free margin, where they breached the weakened epithelial barrier. Further analyses demonstrated that these immune cells enter the ventricles via anatomically specialized “hotspots” at the distal tips of ChP villi. In newborn mice, ChP-driven inflammation drives brain malformations reminiscent of MIA phenotypes reported by others. Thus, the developing ChP both mounts an inflammatory response and provides a gateway for immune cell entry into the brain.
Taken together, our work provides a new way of seeing the ChP and its roles as a source of CSF, as a protective brain barrier, and as a potential mediator and/or therapeutic target in various neurologic diseases.
Jin Cui and Neil Dani are postdoctoral fellows in the lab of Maria Lehtinen at Boston Children’s Hospital.
Frederick Shipley is a recent graduate of the Harvard Biophysics Graduate Program, working jointly in the labs of Maria Lehtinen at Boston Children’s Hospital and Mark Andermann at Beth Israel Deaconess Medical Center.
To learn more in the original research articles:
Tracking Calcium Dynamics and Immune Surveillance at the Choroid Plexus Blood-Cerebrospinal Fluid Interface. Shipley FB, Dani N, Xu H, Deister C, Cui J, Head JP, Sadegh C, Fame RM, Shannon ML, Flores VI, Kishkovich T, Jang E, Klein EM, Goldey GJ, He K, Zhang Y, Holtzman MJ, Kirchhausen T, Wyart C, Moore CI, Andermann ML, Lehtinen MK. Neuron. 2020 Sep 21:S0896-6273(20)30655-3. doi: 10.1016/j.neuron.2020.08.024. Online ahead of print.
Inflammation of the Embryonic Choroid Plexus Barrier following Maternal Immune Activation. Cui J, Shipley FB, Shannon ML, Alturkistani O, Dani N, Webb MD, Sugden AU, Andermann ML, Lehtinen MK. Dev Cell. 2020 Oct 5:S1534-5807(20)30722-X. doi: 10.1016/j.devcel.2020.09.020. Online ahead of print.
News Types: Community Stories
