By Myung-Gyu Choi and Yun Zhang
Chemical synapses and gap junction-mediated electrical synapses are two types of synaptic connections that regulate information processing and flow in the nervous system. Electrical synapses play an important role in coupling neuronal activities and defective gap junctions are associated with neurological diseases. Previous studies on synaptic plasticity have been largely focused on chemical synapses. Therefore, we know relatively little of the function and regulation of the plasticity of gap junctions. It has been shown in both the goldfish auditory system and mammalian inferior olive brain structure that the activity of glutamatergic chemical synapses mediated by the NMDA-type receptors can modulate the coupling of the gap junctions in the network, generating the plasticity of electrical synapses localized close to the chemical synapses. However, it is not known whether the gap junction plasticity has any function in learning and what cellular changes give rise to the changes in the coupling of the gap junctions.
We addressed this question in C. elegans, a worm often found in rotten fruits and other decaying organic materials. C. elegans feeds on bacteria in these habitats. One big challenge that C. elegans faces is that there are often pathogenic bacteria in its food source, and they produce smells that are attractive to the worm. Because C. elegans depends on olfactory sensation to find food, the yummy-smelling pathogenic bacteria can be a serious threat. We previously found that C. elegans learns to suppress its preference for the smell of infectious bacteria after ingesting the bacteria for a few hours. This learning behavior is similar to conditioned aversion found in many animals, including humans, through which animals learn to avoid smell or taste of a food that generates stomach distress after ingestion.
Using this learning paradigm, we found that training with pathogenic bacteria reduces the activity coupling of several interneurons that under naive state exhibit correlated response to the smell of pathogenic bacteria. Because the correlated response of these interneurons promotes the locomotion towards attractive odorants, the training-induced decoupling enables the worm to stay away from the pathogen. We further show that the decoupling of the interneurons results from downregulation of the gap junctions that connect these interneurons and is required for the learning to occur. Furthermore, the NMDA-type glutamate receptor expressed in these interneurons downregulates the gap junctions during learning by activating CaMKII, a highly conserved kinase that regulates plasticity of chemical synapses in the mammalian brain. Interestingly, we also found that training with pathogenic bacteria does not alter the activity of major sensory neurons that detect attractive odorants, rather it modulates a nociceptive neuron that signals the smell of pathogenic bacteria as a repulsive cue after training to generate the specificity of the learning.
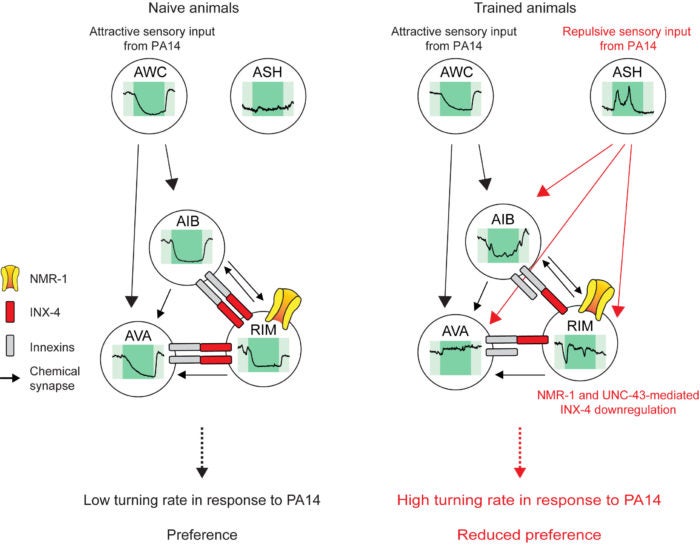
AWC and ASH are sensory neurons that detect attractive and repulsive cues, respectively, and RIM, AIB and AVA are the interneurons connected through chemical and electrical synapses
Therefore, the smell of the training pathogenic bacteria elicits only ‘attractive’ sensory response in naive animals; but induces both ‘attractive’ and ‘aversive’ sensory responses after training. The “mixed” sensory information together with training-induced weakening of the gap junctions decouple the activity of the interneurons, resulting in learned olfactory behavioral response. NMDA receptors and gap junctions are co-localized in the hippocampus and cortical regions of the human brain. In other animals, the modulation of the interactions between chemical and electrical synapses is expected to regulate probabilistic activity of broader circuit areas that may be important for learning and memory. Our study provides the first set of findings that demonstrate the causal role of gap junction plasticity in regulating learning in behavior.
Myung-Gyu Choi is postdoc in the lab of Yun Zhang at Harvard University
Yun Zhang is Professor of Organismic and Evolutionary Biology at Harvard University
Learn more in original research article:
NMDAR-mediated modulation of gap junction circuit regulates olfactory learning in C. elegans. Myung-Kyu Choi, He Liu, Taihong Wu, Wenxing Yang & Yun Zhang Nature Communications volume 11, Article number: 3467 (2020)
News Types: Community Stories
