by Ryann Fame, Morgan Shannon, Maria Lehtinen
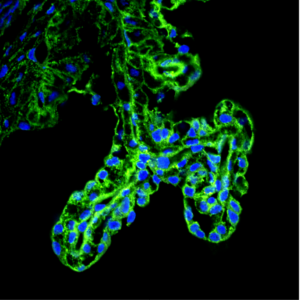
Ciliary body of the eye in 2 month old mice. Blue staining marks cell nuclei; green indicates the cells that will be affected by abnormal expression of the proto-oncogene c-MYC. (Courtesy of Lehtinen Lab)
Missteps during brain development can later lead to the onset of neurologic conditions including brain cancers. In recent years, remarkable progress has been made in identifying cancer-causing “proto-oncogenes.” Although these types of genes may seem inherently “bad,” many are actually essential for normal development and body function. For instance, the proto-oncogene c-MYC is frequently associated with cancers but also required for neural tube closure, an essential step of proper brain development.
We recently discovered that c-MYC levels in the brain decrease immediately after neural tube closure. Using a mouse model, we tested what would happen if c-MYC levels in neural precursor cells were to instead remain high after neural tube closure. We found that persistent c-MYC expression led to tumor formation in two brain tissues specialized for fluid secretion: the choroid plexus, which secretes cerebrospinal fluid into the brain ventricles; and the ciliary body of the eye, which secretes aqueous eye fluid. These tumors were accompanied by large brains and hydrocephalus, a condition of excess fluid in the brain. Notably, more common brain tumors including medulloblastomas or gliomas did not develop.
To identify the types of tumors forming in these tissues, we worked closely with neuropathologists at Boston Children’s Hospital to diagnose these tumors, just as they would be diagnosed clinically in patients. Our mice with artificially high levels of c-MYC in the brain had choroid plexus carcinoma (CPC), a rare and aggressive pediatric tumor. The tumors forming in the eye were diagnosed as ciliary body medulloepitheliomas (CBME). This rare tumor type causes blindness in children.
These findings are exciting because very little is known about the basis of these serious brain cancers, or even how to study CPC and CBME in the lab. Our mouse model is the first ever model of CBME. It is also the first model of CPC with only one gene manipulation. This model recapitulates key features of these types of cancers in patients. Also, using the mouse studies to go back to humans – we conducted additional analyses of patient tumor specimens and found higher c-MYC levels in some CPCs, just like the mice had. This result suggests new ways to characterize and potentially treat CPCs. We hope our research will open avenues to improving future therapeutic interventions for both of these rare and dangerous forms of brain cancer.
To learn more, read the original research article in the American Journal of Pathology:
Shannon ML, Fame RM, Chau KF, Dani N, Calicchio ML, Géléoc GS, Lidov HGW, Alexandrescu S, Lehtinen MK. Mice Expressing Myc in Neural Precursors Develop Choroid Plexus and Ciliary Body Tumors. Am J Pathol. 2018 Jun;188(6):1334-1344.
News Types: Community Stories
