By Christopher Chen
Most of the time we talk about communication between neurons we are referring to the transmission of chemicals across the synapse. When the conversation gets more exotic, we might be referring to an actual physical connection between neurons that allows charge to flow freely between them—often referred to as gap junctions or electrical synapses. In this study, we examined an even less-discussed, third form of communication that is not mediated by chemicals or physical connections: ephaptic coupling.
In principle, ephaptic coupling is quite simple. Because neurons are electrogenic, they produce electric fields. These fields, if strong enough and/or positioned precisely, are able to influence the electrical excitability of neighboring neurons near-instantaneously.
However, ephaptic coupling is notoriously difficult to study. Unlike for example, chemical transmission, there are few manipulations that allow one to specifically and accurately manipulate a neuron’s electric field and its ephaptic coupling. Instead, we very often rely on inference and exclusion to identify ephaptic effects. It is probably for this reason that, while every neuron in principle has the ability to influence others via its electrical field, research in this area is quite sparse.
We looked within the cerebellum at the climbing fiber to Purkinje cell input because it is a classic synapse known for its power and specificity—both good hallmarks for ephaptic effects. The climbing fiber originates in the inferior olive and each one of approximately 7 axon branches wraps around the proximal dendrites of 7 matching Purkinje cells. These climbing fiber to Purkinje cell synapses are excitatory, and the resulting currents are often immeasurably large.
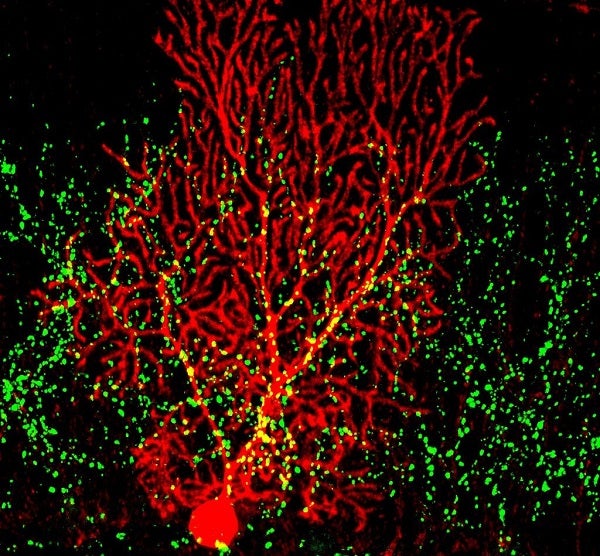
Purkinje Cell in red, and VGlut2 in green. VGlut2 indicates inputs from the climbing fiber
The famous dendrites of the Purkinje cell resemble the sea fans of a coral reef. Purkinje cells are positioned in a single layer with their dendrites parallel to one another. Their somas are spaced only a few microns apart. This densely packed spatial arrangement and the power of the climbing fiber led us to wonder whether the climbing fiber’s input alone could generate an electric field large enough to influence the activity of neighboring Purkinje cells.
Using in vivo and in vitro recording techniques, we could identify the specific inputs from the climbing fiber, and examine its effects on multiple Purkinje cells with submillisecond resolution. Not only was there a huge electrical field change as a result of the climbing fiber input, but it was large enough to affect the excitability of neurons nearly 60 microns away. Using an electrophysiological trick, we could even reverse the direction of this effect on neighboring Purkinje cells by changing the direction of the current elicited by the climbing fiber input, a rare proof of an ephaptic effect. Effectively, we found that a single climbing fiber input could ephaptically influence around 18 neighboring Purkinje cells, and very precisely pause their firing for several milliseconds. Because of the branching of climbing fibers, one would expect over 100 Purkinje cells to be ephaptically and synchronously paused by a single inferior olive action potential. We further found that the precision of this pause may allow this single action potential to effect activity all the way in the forebrain.
These experiments bring to light a fascinating example of ephaptic transmission and how it can change how we think of cerebellum. The climbing fiber input is one of the most powerful excitatory inputs in the brain, and yet it has a widespread and precise inhibitory influence on a swathe of neighboring Purkinje cells. This apparent dichotomy brings a new level of versatility to this classic synaptic input.
Christopher Chen is a postdoctoral research fellow in the laboratory of Wade Regehr at HMS
This story also appears in the HMS Neurobiology Department newsletter, The Action Potential.
Learn more in the original research paper:
Climbing fiber synapses rapidly and transiently inhibit neighboring Purkinje cells via ephaptic coupling. Han KS, Chen CH, Khan MM, Guo C, Regehr WG.
Nat Neurosci. 2020 Sep 7.
News Types: Community Stories
