By Stephanie Rudolph
The cerebellum controls motor coordination, cognitive-affective and autonomic functions. But does it regulate behavior in a sex-specific manner? Several lines of evidence suggest that it might: First, cerebellar dysfunction is associated with sex-biased neuropsychiatric disorders, such as autism, schizophrenia, depression and anxiety. And second, its molecular make-up enables the cerebellum to respond to many sex and context-specific neuromodulators.
For example, cerebellar granule cells, the most numerous neurons in the brain that integrate and relay multisensory and higher-order information from other brain areas, are studded with hormone-sensitive GABA receptors that contain the ? subunit (?GABA). These receptors respond to fluctuations in ambient GABA levels, as well as to stress and sex hormones, and mediate a type of slow inhibition that renders neurons less excitable. In consequence, they critically determine which information travels on, and which information is filtered out. Dysregulation of these receptors is linked to epilepsy, sleep disorders, pain, anxiety, and postpartum depression.
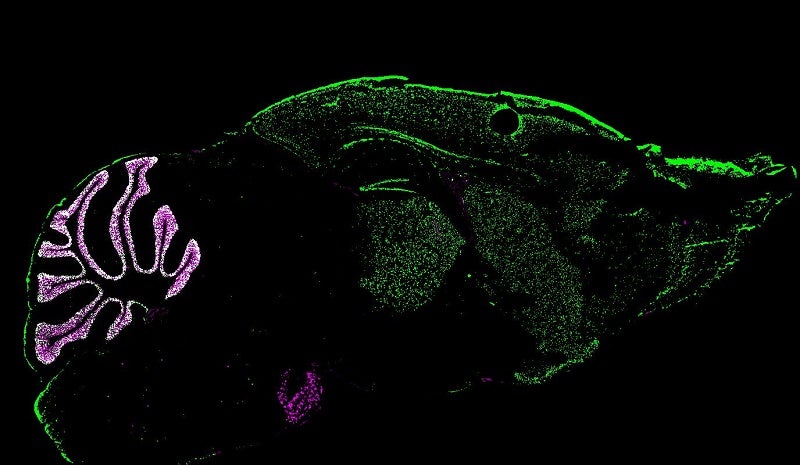
?GABA expression throughout the brain (green) and a cerebellum-specific reporter (red).
Although ?GABA is widely expressed, cerebellar expression by far exceeds that in the rest of the brain. To dissect the cerebellar contribution to the many ?GABA-related behaviors we deleted ?GABA selectively in cerebellar granule cells. Our study found that loss of ?GABA lead to hyperexcitability and GABA insensitivity of granule cells. While locomotion and motor learning were unaffected in cerebellar ?GABA KO animals, we observed a striking increase in stress-related behaviors, especially in female mice. Consistent with these observations, we found female mice to be less interested in social interactions compared to males. The granule cell-specific ?GABA KO females also recapitulated many of the postpartum depression-like behaviors originally observed in female mice that lack the receptor globally.
Why does hyperexcitability of the cerebellar input layer cause such diverse behavioral deficits? As a multisensory structure, the cerebellum communicates with many other brain regions. We found that stress not only disproportionally excited granule cells in ?GABA KO animals, but that it was also associated with altered activation of many cortical and subcortical structures involved in sensory processing, arousal, and anxiety-related behaviors.
With ?GABA emerging as a therapeutic target for postpartum depression our study might encourage exploring ?GABA-focused therapeutic approaches for sex-biased neurodevelopmental and psychiatric disorders that involve the cerebellum.
Stephanie Rudolph is an Assistant Professor in The Dominick P. Purpura Department of Neuroscience and the Department of Psychiatry and Behavioral Sciences at Albert Einstein College of Medicine. She has launched a new lab focused on the cerebellar circuits that control cognition and emotion (view her new website here). She recently completed a postdoctoral fellowship with Wade Regehr.
This story may also appear in the HMS Neurobiology newsletter, The Action Potential.
Learn more in the original research article:
Rudolph, S., Guo, C., Pashkovski, S. L., Osorno, T., Gillis, W. F., Krauss, J. M., Nyitrai, H., Flaquer, I., El-Rifai, M., Datta, S. R., & Regehr, W. G. (2020). Cerebellum-Specific Deletion of the GABAA Receptor δ Subunit Leads to Sex-Specific Disruption of Behavior. Cell reports, 33(5), 108338. https://doi.org/10.1016/j.celrep.2020.108338
News Types: Community Stories
