By Xuan Huang, Kasper Roet, and Clifford Woolf
Phenotypic screening using stem cell-derived human cells is a powerful new tool for drug discovery. By simultaneously measuring the effects of thousands of compounds on disease-specific cellular phenotypes, such as cell death or axon degeneration, we can discover new targets through the screening compounds’ known biological activities and use this as a start to introduce new treatments. In many neurological disorders, neuronal excitability is altered and contributes to disease onset and progression. If a new screening platform can be designed to measure neuronal excitability in a high-throughput manner, we can then expand the drug development process to discover ways to reverse such pathological phenotypes.
Amyotrophic lateral sclerosis (ALS) is a lethal neurodegenerative disorder characterized by loss of adult motor neurons. Increased motor neuron excitability is observed in patients and is associated both with muscle cramps and shorter survival. Recently a potassium channel opener was shown to suppress the hyper-excitability in a clinical trial, but its side effects have resulted in it being withdrawn from the market.
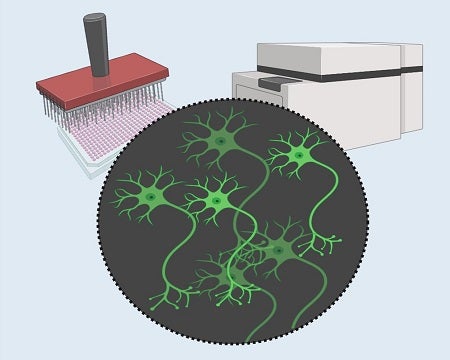
Schematic of GCaMP imaging based high-throughput screening
Collaborating with Pfizer, we created a high-throughput high-content screening platform to identify targets that reduce ALS motor neuron hyper-excitability. While traditional electrophysiological methods are unsuitable for unbiased screens in a large format, we expressed the fluorescent calcium reporter (GCaMP) in neurons to visualize neuronal activities under a microscope in motor neurons differentiated from an ALS patients stem cell line. We tested a library of 2,900 drugs with known annotated actions and after three screening rounds, found 67 compounds that reduced the hyper-excitability of the patient-derived motor neurons. Further bioinformatic deconvolution not only confirmed potassium channels and glutamate receptors as major excitability modulators, but also revealed that type-2 dopamine receptor unexpectedly had an impact on motor neuron excitability. This strategy represents a robust tool for discovering novel targets to regulate abnormal excitability in neurological disorders.
Xuan Huang is a postdoc fellow in the lab of Dr Clifford Woolf.
Kasper Roet is the CEO of QurAlis Corporation and was previously a postdoc fellow in the Woolf lab.
Clifford Woolf is professor of neurology and neurobiology at Harvard Medical School and director of the F.M. Kirby Neurobiology Center at Boston Children’s Hospital.
Learn more in the original research article:
Human amyotrophic lateral sclerosis excitability phenotype screen: Target discovery and validation. Huang X, Roet KCD, Zhang L, Brault A, Berg AP, Jefferson AB, Klug-McLeod J, Leach KL, Vincent F, Yang H, Coyle AJ, Jones LH, Frost D, Wiskow O, Chen K, Maeda R, Grantham A, Dornon MK, Klim JR, Siekmann MT, Zhao D, Lee S, Eggan K, Woolf CJ. Cell Rep. 2021 Jun 8;35(10):109224. doi: 10.1016/j.celrep.2021.109224
News Types: Community Stories
