By Emma West
The retina is the neurosensory part of our eye. As a visual sensor, is a remarkable example of evolution and development. Though often regarded as merely a camera, the retina is much, much more. It is more like a computer using parallel processing to instantly extract meaningful patterns from among the constant rain of photons that impinge on our eyes. The extracted patterns give us valuable information about our environment that is important to our survival and quality of life. To accomplish this type of processing, more than 100 subtypes of retinal neurons exist with specialized functions, cooperating in specialized circuits. Retinal circuits contain combinations of photon-sensing cells, (photoreceptors), interneurons integrating spatial information (bipolar, amacrine, and horizontal cells), and output neurons that send axons to distant brain regions (retinal ganglion cells). Since light coming from each point of our visual scene is focused onto a corresponding point of the retina for processing, the cellular machinery to extract specific features like colors and edges must be tiled across retinal space.
This exceptional spatial organization and vast cellular diversity pose a developmental challenge: how are diverse retinal neurons made at the perfect times and places to integrate into functional circuits throughout the retina? It was known that the major retinal cell types are generated in a conserved order, but very little was known about how the subtypes are made and distributed across space during development. Our study tackled this question, using retinal bipolar interneurons as a model system.
We found that the 15 subtypes of bipolar interneurons are born in an order that reflects their function: subtypes that hyperpolarize in response to light are born before those that depolarize to the same stimuli. Looking closer at their birthdates across space and time, we found that subtype birthdates follow striking spatiotemporal wave patterns. These patterns, and the overall order of subtype genesis, led to a developmental model where subtypes arise from common retinal lineages in a hierarchical order. Interestingly, computational modelling suggested that hierarchical retinal lineages could not only produce the observed spatiotemporal birthdate patterns but could also ensure that neuronal subtypes tile roughly evenly across space.
Examining retinal lineages directly, we found that there is selection against homotypic bipolar cells being made by the same mother cell at the end of retinal development. While this result seems surprising, it is consistent with the hierarchical model and could additionally ensure that each subtype is tiled across space locally.
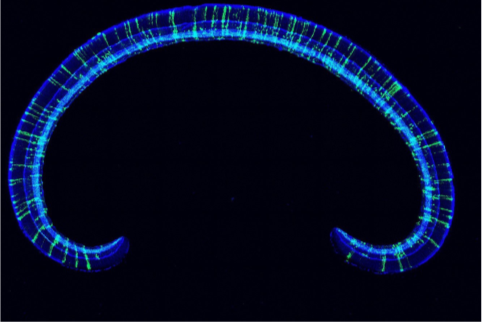
A complete section of the mouse retina is shown. Measuring cellular birthdates and bipolar subtype identities in whole retinal sections revealed striking patterns of subtype development across both space and time that lead to a model of retinal subtype genesis.
This work inspired a model of retinal subtype development. Neuronal subtypes arise in a specific order from retinal progenitor cells and their birthdates are a precise function of space and time. Reframing the developing retina in space-time coordinates will allow us to ask more targeted questions about subtype origins and developmental relationships.
Emma West is a graduate student in the Biological and Biomedical Sciences Program in Connie Cepko’s lab in the Genetics Department.
Learn more in the original research article:
West ER, Lapan SW, Lee C, Kajderowicz KM, Li X, Cepko CL. Spatiotemporal patterns of neuronal subtype genesis suggest hierarchical development of retinal diversity. Cell Rep. 2022;38(1):110191.
News Types: Community Stories
