by Charles Zucker and John Dowling
Globally, macular degenerations of the retina are a leading cause of legal blindness (visual acuity less than 20/200), and little is known about their origin. We describe electron microscopic observations from the retina of a woman afflicted with a rare form of macular degeneration called macular telangiectasia (MacTel). We observed specific changes in mitochondria, the organelle that provides energy-rich molecules to cells. A degeneration of the Müller (glial) cells in the central retina of MacTel patients is known to occur, and the Müller cells synthesize serine, an amino acid necessary for mitochondrial structure and function.
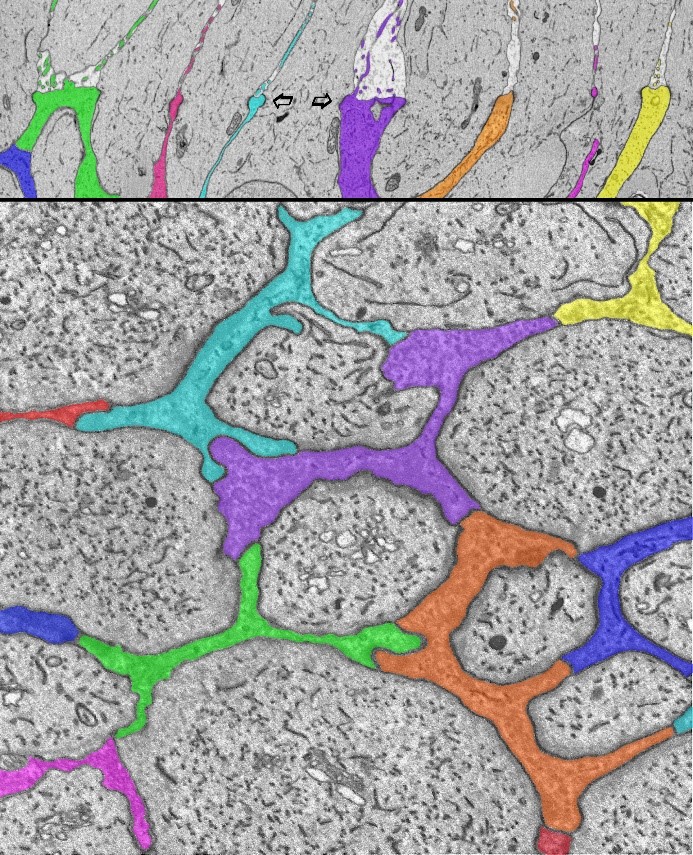
Electron microscope images showing Müller glial junctions in the human retina. Müller cells, the primary glial cell type in the retina, have a varied and complex relationship with retinal neurons. They extend from the inner surface of the retina to the photoreceptors and extend fine microvillus processes into the extracellular space above (upper panel). Seen here at the outer limiting membrane of the human central retina, Müller glial cell processes (colored) surround the inner segments of the photoreceptor cells, and interdigitate with them (arrows), and with each other. This forms a specialized structure with both tight- and adhaerens-junctional properties that constitutes part of the blood-retinal (brain) barrier. These specialized junctions appear in the light microscope as a membrane: hence the name outer limiting membrane. High-throughput serial-section electron microscopy reveals the fine details of that glial/neuronal relationship oriented radially (top, arrows) and horizontally (bottom). Using this connectomics approach, profound changes in these features are identified in a neurodegenerative disease of the retina, Macular Telangiectasia.
The disease is clinically confined mainly to a “MacTel zone” in the central macular region of the retina. We used electron microscopic connectomics techniques, optimized for disease analysis, that allow us to generate high-resolution (4 nm) image data covering large areas of the retina throughout its entire thickness. A major observation showed specific changes in mitochondrial structure in all retinal cell types within and outside the MacTel zone – well beyond the region where the disease manifests clinically! We also identified an abrupt boundary of the MacTel zone that is coincident with the loss of Müller cells and macular pigment. Since Müller cells synthesize serine for uptake by retinal neurons, we propose that a deficiency of serine, required for mitochondrial function, causes mitochondrial changes that underlie MacTel development.
We describe the approach used in this study as “targeted high-throughput connectomics.” For the purposes of learning about pathological mechanisms, where ultimately many samples will need to be analyzed, it is not practical, nor advantageous, to image and reconstruct the entire volume – a more ‘traditional’ connectomics approach. The actual block of tissue examined covers a retinal area of about 2 mm x 3 mm, with a thickness on the order of one third of a millimeter. Slicing the volume into approximately 30 nm thin sections resulted in about 10,000 serial sections collected onto plastic tape with an automated tape-collecting ultramicrotome (ATUM). Lower resolution overview images of the entirety of every 100th section were first acquired. This intermediate step was used for guiding the imaging and locating the actual regions of interest (ROIs) for further high-resolution imaging using either a single-beam or our 61-beam scanning electron microscope. The multibeam instrument allows for a significant increase in imaging throughput.
Looking forward, we will continue to use connectomics methods to analyze the development and progression of neurodegenerative diseases of the retina, including the more common forms of age-related macular degeneration. The transition zone that we have identified in MacTel appears to represent a region where the disease process is progressing, and also where the cellular response to try to ameliorate the degeneration is taking place – this calls for a more comprehensive analysis of this region. Uniquely, we also have an eye from the elderly mother of our first MacTel donor who was also afflicted with MacTel, along with glaucoma. We have initiated a parallel study of this retina to gain a deeper understanding of MacTel, the effects of aging on its development, and the interaction with other diseases such as glaucoma. Such analysis demonstrates the power of connectomics to gain an understanding of neurodegenerative diseases in the retina and throughout the brain.
Charles Zucker is a Staff Scientist in MCB (previously an Associate Professor in Anatomy & Neurobiology, BU School of Medicine).
John Dowling is Gordon and Llura Gund Research Professor of Neurosciences in the department of Molecular and Cellular Biology at Harvard.
Learn more in the original research article:
Zucker CL, Bernstein PS, Schalek RL, Lichtman JW, Dowling JE. A connectomics approach to understanding a retinal disease. Proc Natl Acad Sci U S A. 2020;117(31):18780-18787. doi:10.1073/pnas.2011532117
News Types: Community Stories
