By Nicole Scott-Hewitt
The formation of mature neural circuits requires selective pruning of inappropriate synapses and strengthening of appropriate synaptic connections. This process is critical to normal brain maturation; disruptions are thought to contribute to neurological dysfunction. Exploring the mechanisms underlying synaptic pruning has led to the unexpected discovery that glia, non-neuronal brain cells, contribute to this process. Microglia, brain-resident immune cells, play a critical role in synaptic pruning by targeting axons and synapses for elimination through engulfment of immature or less active inputs. Many different mechanisms of microglial-mediated synaptic elimination have been uncovered recently, however the neuronal signals specifying which synapses are targeted were unknown.
Microglia respond to immune ‘eat me’ and ‘don’t eat me signals’, and we hypothesized that one of these signals could be mediating synaptic recognition and elimination. Phosphatidylserine (PS), a lipid normally found within the inside leaflet of the plasma membrane, can become exposed on the surface of dying cells and debris, triggering recognition by immune cells and subsequent engulfment. Recent studies have revealed that this exposure may also occur locally at specific locations on cells, enabling the removal of subcellular structures while sparing the entire cell.
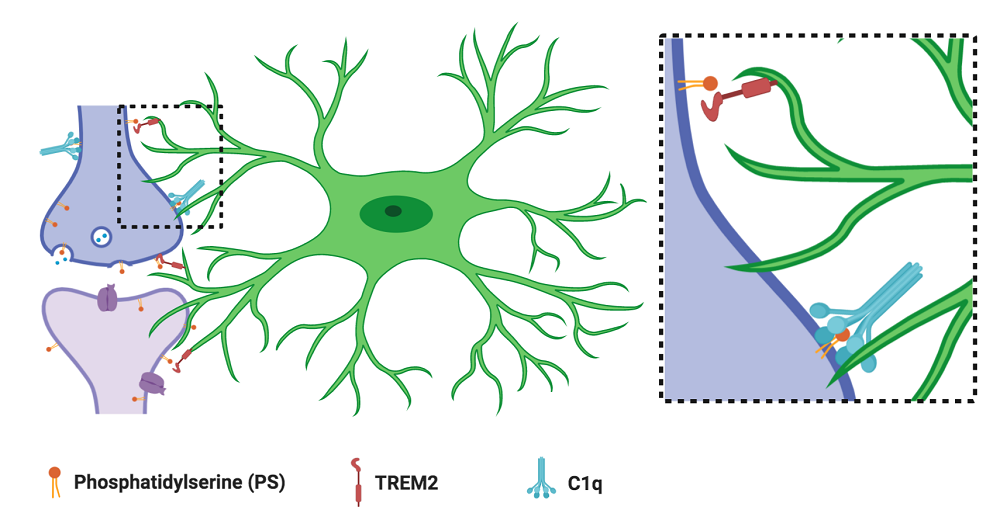
Local synaptic phosphatidylserine (PS) exposure leads to microglial (green) recognition and elimination. The microglial PS receptor TREM2 and the secreted complement protein C1q contribute to this process. Schematic generated using BioRender.
In our current study, we demonstrate that local PS exposure occurs on healthy neurites and synapses in a developmentally regulated manner, contributing to microglial recognition and subsequent elimination. When we isolated neurons and microglia from the brain and cultured them together, we found that microglial-mediated synaptic reductions were dependent on PS and the microglial receptor TREM2, a PS-receptor shown previously to contribute to microglial synaptic pruning. In mice, we found that PS exposure occurred locally at synapses during periods of developmental synaptic pruning, and that microglia engulfed PS-labeled material. Finally, in animals lacking the complement protein C1q, a model previously found to have disrupted pruning, we observed an increase in synaptic PS exposure along with a decrease in microglial PS engulfment. Taken together, our data provide insights into microglial-mediated synaptic pruning by identifying a novel role of locally exposed PS on healthy, developing neurons in instructing microglial engulfment during developmental critical periods.
Nicole Scott-Hewitt is a postdoc in the lab of Beth Stevens at Boston Children’s Hospital.
Learn more in the original research article:
Scott-Hewitt, N., Perrucci, F., Morini, R., Erreni, M., Mahoney, M., Witkowska, A., Carey, A., Faggiani, E., Schuetz, L. T., Mason, S., Tamborini, M., Bizzotto, M., Passoni, L., Filipello, F., Jahn, R., Stevens, B., & Matteoli, M. (2020). Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. The EMBO journal, 39(16), e105380. https://doi.org/10.15252/embj.2020105380
News Types: Community Stories
