By Ken Tao and Brad Lowell
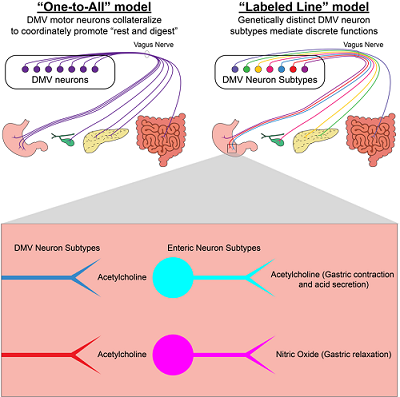
Two different models for how vagal motor neurons coming from the brain may connect to and control digestive system organs. Our work shows evidence for the “labeled line” model where subtypes of DMV neurons innervate distinct regions of the digestive system. The box at the bottom shows what we think is happening with different subtypes of DMV neurons that project to the same organ of the digestive system. They may control separate enteric neuron populations. For instance, we saw a case of opposing actions in the stomach – with one type of DMV neuron controlling acetylcholine neurons responsible for gastric contraction and another controlling nitric oxide neurons responsible for gastric relaxation.
Despite the global rise in metabolic diseases (e.g., obesity, diabetes), there are few effective treatments, largely because of the lack of knowledge on the pathophysiology of these illnesses. The vagus nerve is the information highway between the brain and gut, an important part of the parasympathetic nervous system (PNS), and a clinical target for treating various metabolic disorders. While we know a lot about the anatomy of the vagus nerve, it is still a mystery how its neurons carry messages from the brain to different parts of the digestive system – the stomach, intestines, gallbladder and pancreas. Does each vagal motor neuron coming from the brainstem connect to all of these different regions – which would mean that the brain simultaneously broadcasts to all these sites which collectively cause a broad spectrum of effects? Or are they divided by categories, with one set going to stomach, another to gallbladder and so forth – which would mean that the brain has the dexterity to individually control, as needed, a whole host of different effects? Answering this question is important because the two possible answers have very different implications for how the brain controls the viscera, and this may explain why current treatments involving the vagus nerve lack specificity for the desired outcomes.
The dorsal motor nucleus of the vagus nerve (DMV) is the primary source of parasympathetic input to the digestive system. By using single-cell transcriptomics, we identified seven transcriptionally distinct subtypes of DMV neurons and genetic markers for each subtype. Using recombinase-expressing mouse lines and recombinase-dependent antegrade neural projection tracing techniques, we found two DMV subtypes that exclusively innervate the glandular stomach and do not innervate the nonglandular stomach, small intestine, large intestine, gallbladder, or pancreas. Furthermore, these two DMV subtypes target different enteric neurons that release functionally opposing neurotransmitters (acetylcholine versus nitric oxide). In total, our findings strongly support the “labeled line” model of communication between the DMV and the gut. This discovery reveals that the parasympathetic nervous system utilizes a striking division of labor to control autonomic function, and because of this wiring, has the dexterity to concurrently do very different things in regards to controlling the viscera. By discovering genes that uniquely mark these vagal neurons, and then utilizing corresponding recombinase driver mice which allow experimental access to these neurons, it will now be possible to identify upstream neurons in the brain that are key in regulating each of these different visceral processes – in short, how the brain controls the viscera.
Ken Tao is a PhD student in the lab of Brad Lowell and is co-mentored by Steve Liberles.
Brad Lowell is a Professor of Medicine at Beth Israel Deaconess Medical Center and Harvard Medical School.
Learn more in the original research article:
Highly selective brain-to-gut communication via genetically defined vagus neurons.
Tao J, Campbell JN, Tsai LT, Wu C, Liberles SD, Lowell BB. Neuron. 2021 Jul 7;109(13):2106-2115.e4. doi: 10.1016/j.neuron.2021.05.004. Epub 2021 Jun 1. PMID: 34077742; PMCID: PMC8273126.
News Types: Community Stories
