by Daniel Gilliam and Elizabeth Pollina
The brain is continuously confronted by stimuli from the external world. As neuronal circuits process these experiences, they undergo changes in how the cells within the circuit are wired together. These changes are broadly described as ‘neuronal plasticity’ and are mediated in part by changes to neuronal gene expression in the wake of synaptic activity. Despite these rapid changes in gene expression being necessary for aspects of plasticity, elevated synaptic activity triggers significant DNA damage while activating these inducible genes. Over time this DNA damage burden threatens to corrupt the underlying genetic sequence of these essential activity-regulated genes. How then do neurons coordinate a response to the DNA damage from synaptic activity? How do neurons protect the genes they rely on for plasticity from this accumulating DNA damage?
We have identified a new DNA repair signaling pathway embedded within the neuronal response to stimulation, via activity-dependent interaction of the inducible transcription factor NPAS4 with the NuA4 chromatin modifier/DNA repair complex. This molecular complex helps to assemble specific DNA repair machinery at the right place and time following neuronal activity, just as the inducible genes bound by this complex confront DNA damage. Several components of this complex have already been implicated in neurodevelopmental and autism spectrum disorders.
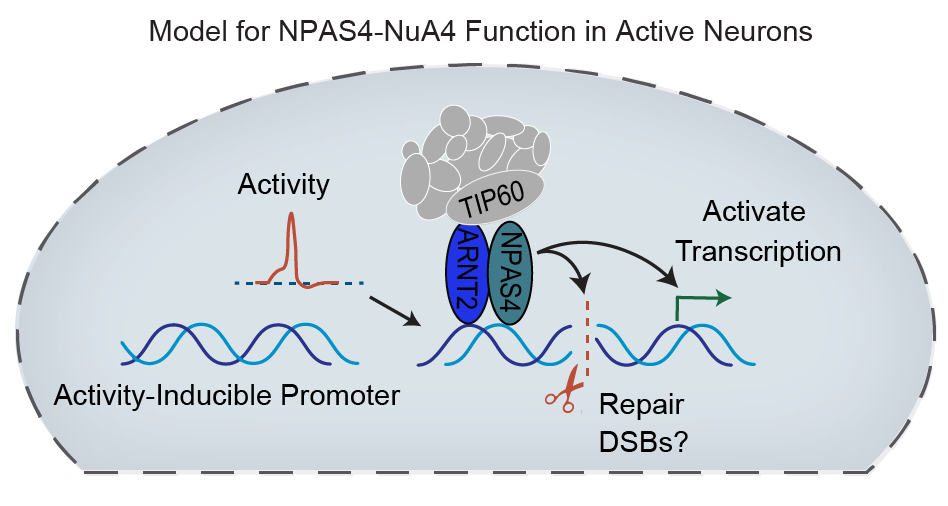
Model showing how NPAS4-NuA4 may be working in stimulated neurons, activating stimulus-dependent transcription while simultaneously coordinating DNA break repair.
The uniquely neuronal factor NPAS4 now sits at the nexus of several essential signaling pathways and appears to be necessary for achieving a normal lifespan in mice. Many questions remain about the relationship between synaptic activity and neuronal longevity, and the NPAS4-NuA4 complex will be an essential entry point for studying ageing and neurodegenerative diseases in humans.
See also: A New Strategy for Repairing DNA Damage in Neurons, an article in HMS News.
Daniel Gilliam is a graduate student in the Program in Neuroscience, conducting research in the lab of Michael Greenberg in the Department of Neurobiology at Harvard Medical School.
Elizabeth Pollina carried out this research as a postdoctoral fellow in the Greenberg lab and is now an assistant professor of developmental biology at the Washington University School of Medicine.
Learn more in the original article:
A NPAS4-NuA4 complex couples synaptic activity to DNA repair
Pollina EA, Gilliam DT, Landau AT, Lin C, Pajarillo N, Davis CP, Harmin DA, Yap EL, Vogel IR, Griffith EC, Nagy MA, Ling E, Duffy EE, Sabatini BL, Weitz CJ, Greenberg ME.. Nature. 2023 Feb;614(7949):732-741.
News Types: Community Stories
