By Akiko Terauchi and Hisashi Umemori
Dopamine in the brain regulates many functions, including movement and motivation. Movement and motivation are regulated by two distinct dopaminergic pathways in the brain called the nigrostriatal and mesolimbic pathways. The nigrostriatal pathway transmits dopaminergic signals from the substantia nigra pars compacta (SNc) to the dorsal striatum (caudate-putamen [CPu]). This pathway controls movement, and defects in this pathway are linked to Parkinson’s disease. By contrast, the mesolimbic pathway transmits dopaminergic signals from the ventral tegmental area (VTA) to the ventral striatum (nucleus accumbens [NAc]). This pathway controls motivation, and its defects are linked to schizophrenia and depression. Therefore, these two dopaminergic pathways must be appropriately established during development for the optimal functioning of the brain. However, how these discrete dopaminergic pathways are established was unknown.
To address this question, we first characterized how these dopaminergic pathways are established. We found that there are two distinct factors released from the respective target of each pathway (i.e., CPu or NAc) that specifically organize the nigrostriatal vs. mesolimbic connection. We then performed an unbiased screen and identified that two groups of TGFβ (Transforming Growth Factor β) family members, BMP6/BMP2 and TGFβ2, from the target regulate nigrostriatal and mesolimbic pathways, respectively. Downstream in dopamine neurons, two distinct signaling molecules, Smad1 and Smad2, are activated and required for the establishment of nigrostriatal vs. mesolimbic pathways. We proved their roles using mutant mice, by performing histology, electron microscopy, and physiology both in vitro and in vivo. Remarkably, we further found that the inactivation of Smad1 and Smad2 in dopamine neurons affects specific mouse behaviors: Smad1 mutant mice show motor defects, which is consistent with the role of the nigrostriatal pathway in movement, and Smad2 mutant mice show a lack of motivation, which is consistent with the role of the mesolimbic pathway in motivation.
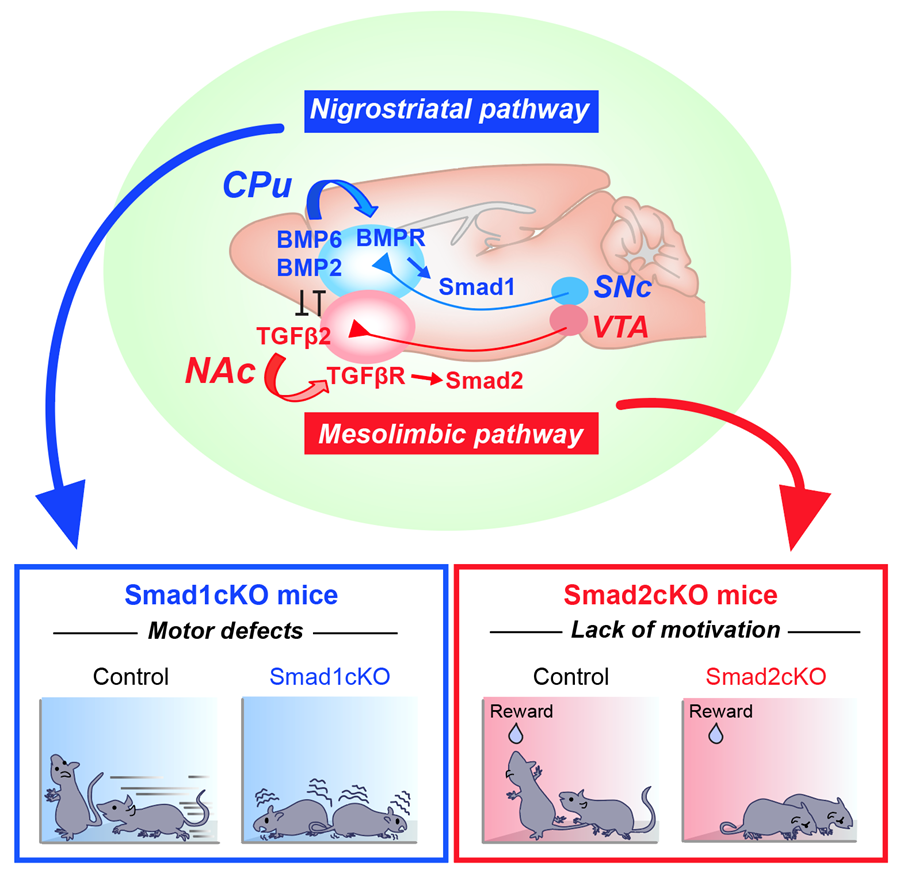
The molecular logic being utilized in the brain to establish parallel, but functionally discrete dopaminergic pathways. (Top) BMP6/2–BMP receptor (BMPR)–Smad1 and TGFβ2–TGFβ receptor (TGFβR)–Smad2 axes establish nigrostriatal vs. mesolimbic synapses. BMP6/2 and TGFβ2 antagonize each other to further strengthen the specificity. (Bottom) Smad1 mutant mice show motor defects and Smad2 mutant mice show a lack of motivation.
These findings reveal how functionally segregated dopaminergic pathways are established in the mammalian brain by specific molecular actors. An additional interesting aspect we showed is that the BMP–Smad1 and TGFβ–Smad2 signaling pathways antagonize each other, which strengthens the specific segregation of nigrostriatal and mesolimbic pathways.
Finally, because Smads are involved in the development and maintenance of dopaminergic pathways, our work may provide strategies to treat relevant, pathway-specific disease symptoms, such as motor defects in Parkinson’s disease and avolition in schizophrenia and depression, by targeting specific BMPs/TGFβ and/or Smads, without affecting the other pathway.
Akiko Terauchi is an Instructor in Neurology at the F.M. Kirby Neurobiology Center at Boston Children’s Hospital.
Hisashi Umemori is a Professor of Neurology at Harvard Medical School and the F.M. Kirby Neurobiology Center at Boston Children’s Hospital.
Learn more in the original research article:
The projection-specific signals that establish functionally segregated dopaminergic synapses
Terauchi A, Yee P, Johnson-Venkatesh EM, Seiglie MP, Kim L, Pitino JC, Kritzer E, Zhang Q, Zhou J, Li Y, Ginty DD, Lee WA, Umemori H. Cell. 2023 Aug 31;186(18):3845-3861.e24. doi: 10.1016/j.cell.2023.07.023. Epub 2023 Aug 16. PMID: 37591240; PMCID: PMC10540635.
News Types: Community Stories
