By Joshua Sanes
Damage to our brain or spinal cord is generally irreversible, leading to a gloomy prognosis for neurodegenerative disease or traumatic injury. Our group in MCB and the Center for Brain Science, and that of Zhighang He in the Kirby Center at Boston Children’s Hospital, are two among many that have attempted to make a dent in this hard problem.
We used the mouse retina in our collaborative work. Neurons called retinal ganglion cells (RGCs) send axons from the retina to the rest of the brain through the optic nerve. When the optic nerve is crushed, most RGCs die and few if any survivors regrow new axons. Can the fraction of survivors be increased? Can rescued cells be coaxed to regenerate their axons?
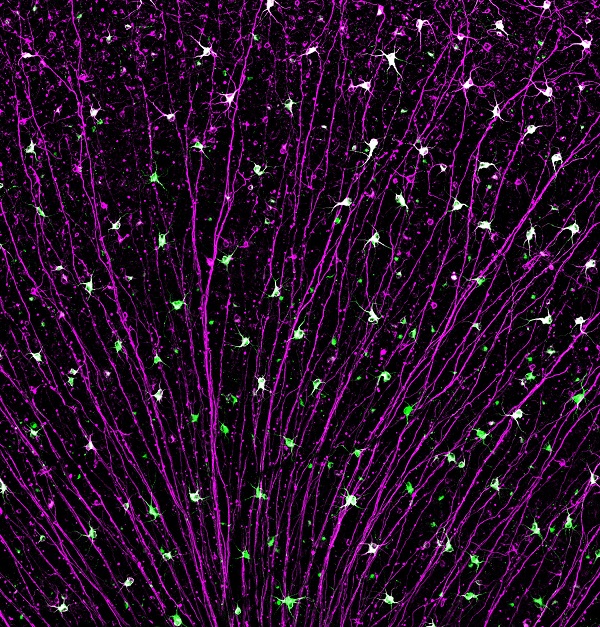
Portion of a mouse retina showing surviving retinal ganglion cells following injury to the optic nerve. Their axons (purple), converge on the optic nerve. Cell bodies of a particularly resilient neuronal type are stained in green.
In our latest work, we examined genes that affect survival and regeneration. In one of two papers (Jacobi et al., Neuron, 2022), we identified three gene expression programs activated by injury. One appears to result in neuronal death, a second in neuronal survival and the third in axon regeneration. When axons are damaged, all three programs are activated, but the death program persists while the other two are quickly extinguished. In contrast, when the retinas had been treated in ways that the He lab previously showed can improve survival and promote regeneration (albeit not to a clinically useful extent), the death program is extinguished but the survival and regeneration programs persist. The implication is that enhancing expression of survival and regeneration genes could improve these qualities – an idea they validate for a few candidates.
In the companion paper we focused on the death pathway (Tian et al. 2022). We performed an in vivo screen of 1893 transcription factors, deleting them (using CRISPR technology) one at a time and assessing survival of injured RGCs. We found several cases in which deletion of a transcription factor enhanced survival, implying that the factor is normally part of the death program. Combining these results with those from a survey of epigenomic changes that accompany RGC injury, we converged on a set of four related master regulators of the choice between survival and death. Indeed, deleting these genes enhanced survival not only following injury but also in a model of glaucoma, a prevalent disease in which blindness results from RGC dysfunction and death.
Importantly, mechanisms discovered in the optic nerve crush model can illuminate processes that underlie injuries and diseases elsewhere in the central nervous system. We are eager to find out if this will be true for the new mechanisms and targets we have identified.
Joshua Sanes is Jeff C. Tarr Professor of Molecular and Cellular Biology at Harvard University.
Learn more in the original research articles:
Jacobi A, Tran NM, Yan W, Benhar I, Tian F, Schaffer R, He Z, Sanes JR.
Overlapping transcriptional programs promote survival and axonal regeneration of injured retinal ganglion cells. Neuron. 2022 Jun 24:S0896-6273(22)00540-2.
Tian F, Cheng Y, Zhou S, Wang Q, Monavarfeshani A, Gao K, Jiang W, Kawaguchi R, Wang Q, Tang M, Donahue R, Meng H, Zhang Y, Jacobi A, Yan W, Yin J, Cai X, Yang Z, Hegarty S, Stanicka J, Dmitriev P, Taub D, Zhu J, Woolf CJ, Sanes JR, Geschwind DH, He Z.
Core transcription programs controlling injury-induced neurodegeneration of retinal ganglion cells. Neuron. 2022 Jun 24:S0896-6273(22)00541-4.
News Types: Community Stories
