By Chelsey Derderian-LeBlang and Rosalind Segal
The study of human biology and disease has been revolutionized by the creation of induced pluripotent stem cell (iPSC) models. iPSCs are generated from human skin and blood cells and can then be turned into various cell-types of interest, allowing scientists to observe and manipulate human cell populations that were previously inaccessible in a dish. In recent years, scientists have developed multiple methods to create iPSC-derived somatosensory neurons (idSNs), the cells that convey touch and pain sensations from the environment to the brain. These models help scientists understand sensory biology and the underlying mechanisms of diseases like neuropathy and chronic pain, which impact millions of people world-wide. Importantly, these human cells are increasingly being used to develop and test therapeutics for sensory nerve disorders. Indeed, new directives from the FDA emphasize the use of human models in drug development.
While the current human sensory neuron models provide an accessible system for scientists to study sensory biology, the cells produced are similar to developing embryonic sensory neurons rather than mature neurons. Therefore, conclusions from current studies may not yield results that are directly relevant to the physiology of adult patients. In our latest research, we created a new method to advance the maturation of iPSC-derived somatosensory neurons, aiming to improve models to understand and treat sensory disorders.
In the developing embryo, support cells called satellite glia surround the cell bodies of somatosensory neurons, just as these neurons begin to take on their adult shape. This intimate relationship is maintained throughout adulthood, and this allows satellite glia to regulate sensory neuron function. We hypothesized that exposure of idSNs to external cues from satellite glia would accelerate their maturation during the differentiation process, producing a more physiologically relevant model of human sensory neuron biology. To test our hypothesis, we developed a new differentiation protocol in which immature idSNs are co-cultured with embryonic rat satellite glia (rSGs) during their final maturation period.
To determine the impact of satellite glial co-culture on idSN maturation, we evaluated idSN morphology, physiology, and gene expression. We found that idSNs matured alone present primarily with embryonic morphology, but idSNs differentiated in co-culture transition to a mature, T-shaped, pseudounipolar morphology. Pseudounipolar morphology allows for the regulation of communication via electrical signals between the peripheral skin and muscle and the central nervous system. Through analysis of neuronal electrical activity, we found that co-cultured idSNs fire significantly more action potentials than idSNs matured alone, functioning on par with sensory neurons in living rodents. These changes in morphology and physiology are supported by an increase in expression of genes related to neuronal differentiation, axonal development, and action potential firing.
We next asked how satellite glia contribute to the development of sensory neurons. We found that development of pseudounipolar morphology in idSNs depends on physical contact between satellite glia and neurons. Additionally, we found that a contact-dependent signaling pathway, involving semaphorin and plexin proteins, contributes to development of pseudounipolar morphology in cocultured idSNs. This is likely to be just one of several such cues.
Lastly, we tested whether our new protocol provides an improved model of human sensory neuropathy. More than half of cancer patients treated with some chemotherapies develop chemotherapy-induced peripheral neuropathy (CIPN), with pain and sensory disturbances due to degeneration of sensory nerve axons. So, we assessed the impact of chemotherapy treatment on neurodegeneration in both cocultured idSNs and idSNs that were matured alone. We found that cocultured idSNs are significantly more susceptible to chemotherapy-induced neurodegeneration compared to idSNs matured alone. These results suggest that satellite glia may contribute to the axonal degeneration observed in CIPN.
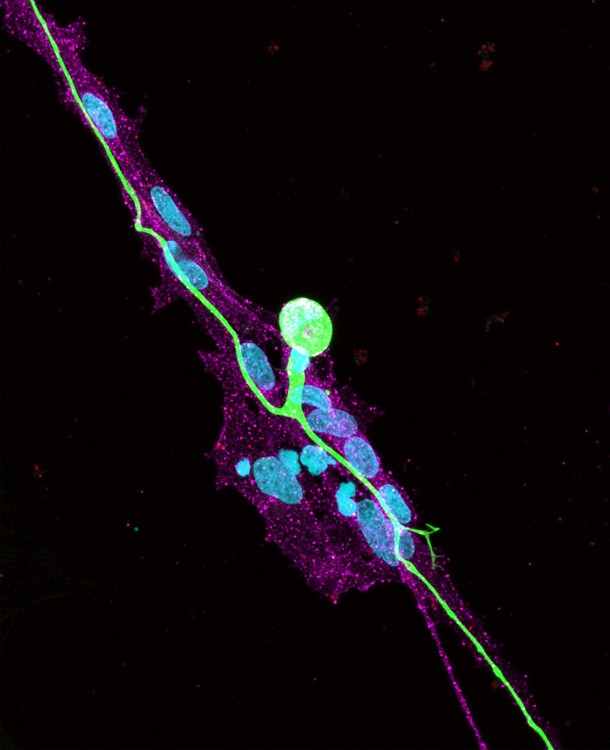
Human iPSC-derived sensory neurons (green) matured in contact with rodent satellite glia (magenta) develop mature, T-shaped, pseudounipolar morphology. All cell nuclei are shown in blue.
Overall, our study provides an accessible and improved protocol for scientists to model human sensory neuron development, health, and disease. We have gained new insights into the role of satellite glial cells in the development of sensory neurons and identified a role for satellite glia in chemotherapy-induced neurodegeneration. We are now working on a protocol to generate human iPSC-derived satellite glia—which together with the new and improved idSNs would allow us to build a more biologically human coculture system, increasing the physiological relevance of future research.
Chelsey Derderian-LeBlang is currently a postdoctoral fellow in the Segal Lab within the departments of Neurobiology at Harvard Medical School and Cancer Biology at the Dana Farber Cancer Institute.
Rosalind Segal is a Professor of Neurobiology at Harvard Medical School.
Learn more in the original research article:
Satellite glial contact enhances differentiation and maturation of human iPSC-derived sensory neurons.
LeBlang CJ, Pazyra-Murphy MF, Silagi ES, Dasgupta S, Tsolias M, Miller T, Petrova V, Zhen S, Jovanovic VM, Castellano D, Gerrish K, Ormanoglu P, Tristan CA, Singeç I, Woolf CJ, Tasdemir-Yilmaz O, Segal RA. . Stem Cell Reports. 2025 Oct 14;20(10):102639. doi: 10.1016/j.stemcr.2025.102639. Epub 2025 Sep 18.
News Types: Community Stories
