By Adam E Cohen and Pojeong Park
Many neurons have beautiful and elaborate dendritic trees, where the neuron receives synaptic inputs. In the classical view of a neuron, these inputs propagate passively to the cell body, and if the inputs cross a threshold, the neuron produces an electrical spike which travels down the axon and then stimulates the next neurons in the chain. The spike also propagates back up the dendrites (a “back-propagating action potential”) and interacts with the synaptic inputs to make synapses stronger or weaker, i.e. to drive synaptic plasticity.
Careful electrical measurements of dendrites using tiny glass capillaries (“patch pipettes”) have shown that the picture is more complex. Dendrites can be triggered to produce several types of electrical spikes on their own, distinct from the spikes made at (or near) the cell body. What are these dendritic spikes for?
For the past fifteen years, our lab has been developing tools to image bioelectrical signals — to convert bioelectrical fluctuations into visible flashes of fluorescence. We discovered that some microbial rhodopsin proteins, derived from various exotic microorganisms, had the strange property that their fluorescence was exquisitely sensitive to electrical signals. We and others have worked hard to engineer these proteins to be fast, bright, and sensitive reporters of voltage; and to develop gene expression strategies, microscopes, and software to coax as much information as possible from these little flickering beacons.
When Pojeong joined our lab, he suggested that by mapping bioelectrical signals throughout a dendritic tree, we might gain insights into the role of dendritic excitations in neural information processing. We started with two simple hypotheses. 1: Maybe the excitations played a role in conveying synaptic inputs to the cell body. For example, if a dendrite received strong enough synaptic excitation, perhaps that would trigger a local dendritic spike, which could then amplify the effect of those inputs at the cell body. 2: Maybe the excitations played a role in back-propagation. For example, perhaps the right pattern of synaptic inputs and back-propagating action potentials could trigger these dendritic spikes, and the spikes might play a role in mediating synaptic plasticity. While these two hypotheses aren’t necessarily mutually exclusive, we guessed that only one would be correct. We reasoned that the underlying computations are totally different for dendritic integration vs. backpropagation-mediated plasticity, yet both processes involve electrical signals passing the same ensemble of dendritic ion channels. It’s hard to imagine how a single set of excitability rules could mediate both processes.
Another postdoc in the lab, David Wong-Campos, built a sophisticated optical system to record minuscule flickers of red fluorescence from dendrites in intact brain tissue. David’s optical system could also deliver targeted flashes of blue light to regions on a dendrite, and Pojeong expressed in the neurons a blue light-activated ion channel along with the red light-excited voltage indicator. Together, these tools let us tickle the neuron in almost any pattern of space and time, and then map the complete bioelectrical response across the whole dendritic tree. We expressed the molecular tools in a sparse subset of pyramidal cells in the mouse hippocampus, prepared acute brain slices, and started to explore neuronal input-output functions.
It immediately became clear that membrane voltage is quite “stiff”, that is, the voltage tends to spread far and fast throughout the dendritic tree, almost like a broadcast signal. The neuron could be pretty well described by two voltages, one near the cell body and nearby dendrites, and a second voltage out in the far-away dendrites. Pojeong stimulated the neurons in many patterns of space and time, and then used different drugs to block specific ion channels to see how this affected electrical signal propagation in the dendritic tree.
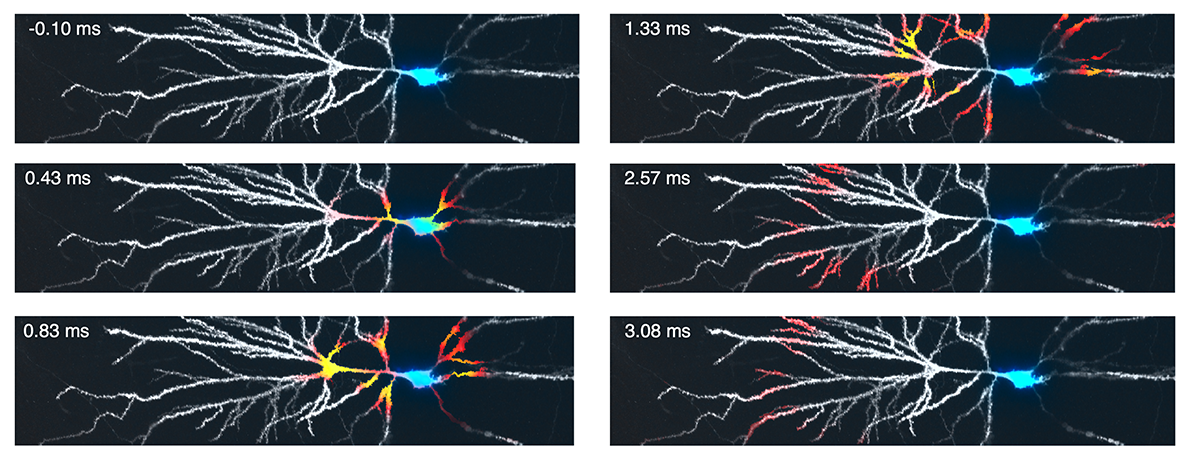
A clear picture emerged from all these experiments. Dendritic excitations were much more readily triggered by back-propagating action potentials than by synaptic inputs alone. Further, the dendritic excitations formed a hierarchy, with easily triggered and fast ones triggering slower-starting and longer-lasting ones, much as smaller sticks ignite larger ones in a well-constructed campfire. Sodium spikes were like kindling, fast and powerful, but quickly extinguished. These sometimes triggered calcium spikes, which were like mid-size sticks, burning for longer before becoming exhausted. Finally, if the pattern of synaptic inputs was just right, the calcium spikes could combine with so-called NMDA spikes, to drive large and sustained dendritic spikes, like the burning of a big log. Back-propagating action potentials coming from the cell body acted as the matches to trigger the dendritic fire, and dendritic potassium channels acted like moisture, suppressing the dendritic fire until they were inactivated.
Based on this picture, we think dendritic excitations play a key role in mediating plasticity. They arise when the right pattern of back-propagating spikes collides with the right pattern of synaptic inputs. Big dendritic spikes drive a lot of calcium influx, and these events are known to be necessary for some forms of learning in behaving animals. Our next step will be to map voltage in dendrites of behaving mice.
Adam Cohen is in the Departments of Chemistry and Chemical Biology, and Physics.
Pojeong Park was a postdoc in Adam’s lab, and now runs his own lab in the Department of Brain Sciences at Daegu Gyeongbuk Institute of Science and Technology, Korea.
Learn more in the original research article:
Dendritic excitations govern back-propagation via a spike-rate accelerometer.
Park P, Wong-Campos JD, Itkis DG, Lee BH, Qi Y, Davis HC, Antin B, Pasarkar A, Grimm JB, Plutkis SE, Holland KL, Paninski L, Lavis LD, Cohen AE. Nat Commun. 2025 Feb 4;16(1):1333.
News Types: Community Stories
