By Maria Sundberg and Mustafa Sahin
Recent intensive studies of the genetic causes of autism and schizophrenia have revealed an association of deletions or duplications of specific chromosomal loci 16p11.2 with these neurodevelopmental and psychiatric disorders. This region is located in chromosome 16, and it contains 29 protein coding genes, and most of them are expressed in the brain. Clinical studies have shown that deletion of 16p11.2 leads to increased brain volume, autism, intellectual disability and severe developmental deficits. In contrast, patients with duplication of the 16p11.2 locus have decreased brain volume and increased risk for developing schizophrenia, depression, bipolar disorder, and autism. Previous studies have also shown that deficits in dopamine signaling lead to problems in social interactions and behavioral difficulties in patients with autism and schizophrenia. However, the molecular mechanisms causing dopaminergic neuron dysfunction in these neurodevelopmental disorders have remained largely unknown.
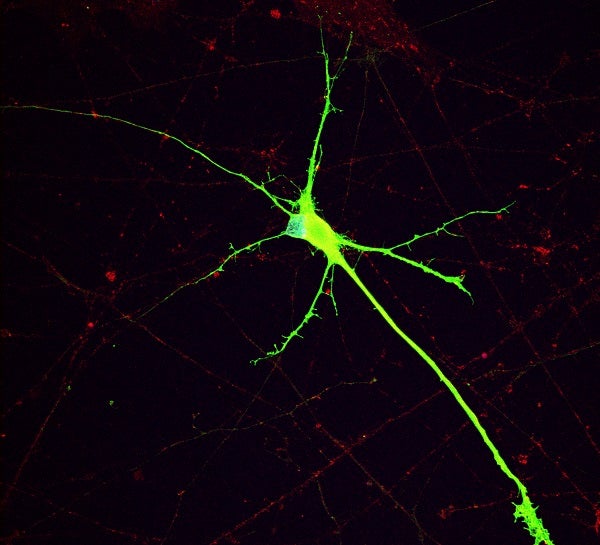
Human iPSC derived dopaminergic neuron with 16p11.2 deletion. The neuron expresses tyrosine hydroxylase (TH, green, a marker of dopaminergic neurons) and synaptophysin (SYP, red, a marker of synaptic vesicles).
To study the effects of 16p11.2 copy number variations on dopamine signaling we differentiated human induced pluripotent stem cells, with either a 16p11.2 duplication or deletion, into dopaminergic (DA) neurons in a cell culture. Then we characterized the molecular and functional phenotypes of these neurons compared to healthy control neurons. We performed RNA sequencing to analyze the transcriptional gene expression changes in the DA neurons. We assessed network activity using both high-density micro-electrode array (MEA) and multi-well low-density MEA platforms.
We found that genes associated with synaptic development and neuronal differentiation were expressed at higher levels in the DA neurons with 16p11.2 deletion. These neurons were hyperexcitable and had increased network firing activity compared to control neurons. Importantly, we identified the RhoA pathway as a potential cause for these network dysfunctions. RhoA (Ras homolog family member A) is a GTPase protein that binds GDP and GTP and regulates activation of various downstream pathways in the cells, and regulates building of actin cytoskeleton, cell division and migration. Previously it has been shown that Rho family of GTPases have an important role in neuronal development, including neurite differentiation and outgrowth, myelination, formation of dendritic spines and axon development.
In this study we demonstrated that the RhoA inhibitor, Rhosin, rescued the hyperactivity and bursting phenotypes of the dopaminergic neuron networks carrying a 16p11.2 deletion. Our study indicates that the RhoA pathway and its inhibitors may serve as a potential treatment target for the neurodevelopmental disorders associated with 16p11.2 deletion.
Maria Sundberg is a post-doctoral research fellow in the Sahin-Lab, at the department of Neurology at the Boston Children’s Hospital.
Mustafa Sahin is Professor in Neurology at Boston Children’s Hospital and Harvard Medical School.
Learn more in the original research article:
16p11.2 deletion is associated with hyperactivation of human iPSC-derived dopaminergic neuron networks and is rescued by RHOA inhibition in vitro.
Sundberg M, Pinson H, Smith RS, Winden KD, Venugopal P, Tai DJC, Gusella JF, Talkowski ME, Walsh CA, Tegmark M, Sahin M. Nat Commun. 2021 May 18;12(1):2897. doi: 10.1038/s41467-021-23113-z. PMID: 34006844; PMCID: PMC8131375.
News Types: Community Stories
