By Yusuke Fukuda
Social distancing has drastically changed our lives, and we are now highly dependent on the various delivery services that provide many of our daily essentials right to our door. In the cell, the essential delivery of cellular components is primarily mediated by motor protein called kinesins, which walk along a tubular track in a step-by-step manner. The distance traveled by kinesins ranges from 10-20 μm in a typical cell and up to a meter in peripheral nerves such as sciatic nerves, which contain the axons of sensory and motor neurons that innervate the tips of our toe. In people, mutations in kinesins cause Charcot-Marie-Tooth (CMT) disease, with neurodegeneration of peripheral nerves leading to progress impairment of motor and sensory functions. Therapeutic opportunities for CMT are currently lacking because of the gap in understanding how pathogenic mutations compromise cargo delivery to axons.
Mutations in KIF5A, one of the 45 kinesin genes, are frequently implicated in CMT, suggesting that KIF5A kinesin motor may have specialized functions in peripheral nerves. In our current study, we identify a RNA-binding protein called SFPQ (Splicing factor Proline and Glutamine rich) that selectively binds to KIF5A rather than other kinesin members. Intriguingly, cultured sensory neurons expressing a mutated SFPQ, with a single amino acid change that prevents binding to KIF5A, have impaired transport of SFPQ-RNA granules and this results in axon degeneration, the same pathology observed in CMT patients. Thus, KIF5A-mediated delivery of SFPQ and its bound mRNAs such as bclw, which encodes a protein implicated in axon survival, protect axons from degeneration. Finally, we show that introducing a synthetic peptide of Bclw into axons, which bypasses the need for mRNA delivery and protein translation, protects axon degeneration in CMT models. These data reveal therapeutic potential in targeting KIF5A-mediated SFPQ transport pathway to treat CMT.
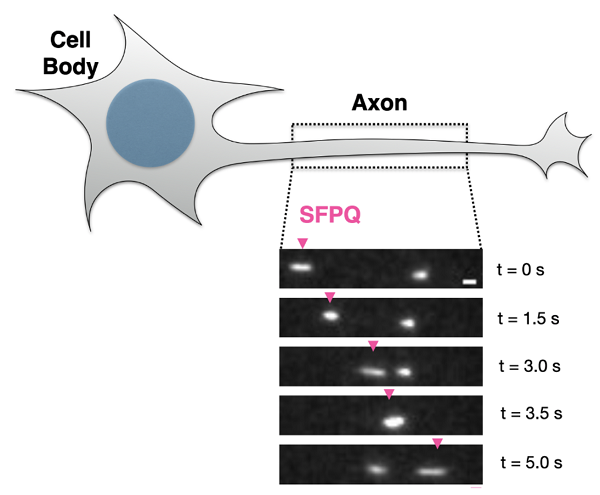
Real time imaging of SFPQ in axons of sensory neurons. SPFQ moves in both directions. Kinesin-mediated transport is shown with pink arrowhead
This work was accomplished in collaboration with many groups including Drs. Loren Walensky and Jarrod Marto at Dana-Farber Cancer Institute and Dr. Lisa Goodrich at Harvard. These studies have broad implications for degenerative diseases beyond CMT, as human mutations in KIF5A and in SFPQ also cause amyotrophic lateral sclerosis (ALS), and these components have also been implicated in hearing loss and in Alzheimer’s disease. Future studies aim to uncover the relevance of KIF5A-mediated transport of SPFQ in other neurodegenerative diseases and explore the use of Bclw peptide as a broadly applicable therapeutic modality.
Yusuke Fukuda recently completed a postdoctoral fellowship in the lab of Rosalind Segal at Harvard Medical School and Dana Farber Cancer Institute. He is now working at Takeda.
This article will also be featured in the HMS Neurobiology Newsletter, The Action Potential.
Learn more in the original research article:
Binding and transport of SFPQ-RNA granules by KIF5A/KLC1 motors promotes axon survival.
Fukuda Y, Pazyra-Murphy MF, Silagi ES, Tasdemir-Yilmaz OE, Li Y, Rose L, Yeoh ZC, Vangos NE, Geffken EA, Seo HS, Adelmant G, Bird GH, Walensky LD, Marto JA, Dhe-Paganon S, Segal RA.
J Cell Biol. 2021 Jan 4;220(1):e202005051.
News Types: Community Stories
