By Adam Granger
Acetylcholine is an important neurotransmitter in the brain that controls learning and memory, alertness, and our ability to maintain attention. In the cerebral cortex, acetylcholine activates downstream neurons to process important incoming stimuli and stimulates changes in synaptic connections to improve the brain’s response in the future. Typically, acetylcholine in the cortex originates from deep-brain neurons that send their projections across large regions of the cortex. However, there is also a small population of local interneurons in the cortex that express ChAT, an enzyme that synthesizes acetylcholine, suggesting an alternative local source.
In addition to acetylcholine, these ChAT-expressing interneurons also synthesize and release the neurotransmitter GABA. Neurons that release multiple neurotransmitters are a poorly understood population of signaling neurons in the brain. This phenomenon is especially confusing in the case of neurons that release both acetylcholine and GABA, because GABA is typically an inhibitory neurotransmitter and acetylcholine is a typically excitatory one. We therefore wanted to understand why these ChAT-expressing interneurons would release two different neurotransmitters with opposite effects on downstream neurons.
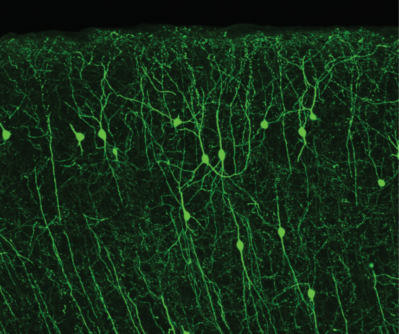
GFP in cortical neurons expressing ChAT, an enzyme necessary for making the neurotransmitter acetylcholine.
One hypothesis is that these neurons release acetylcholine and GABA onto different types of downstream neurons. To test this hypothesis, we first confirmed that this ChAT-expressing interneuron population expressed all of the necessary genes and proteins to release both acetylcholine and GABA. We then used optogenetics to activate these neurons with blue light while recording electrical currents from downstream neurons that potentially received their synaptic input. We found that these neurons consistently release GABA onto inhibitory subtypes of cortical neurons, while also releasing acetylcholine much more sparsely onto other downstream ChAT-expressing interneurons. We also were able to show that only a subset of the pre-synaptic terminals (the site from which they release neurotransmitters) of the original ChAT-expressing interneurons were able to release acetylcholine.
These results show that these neurons are able to specialize which neurotransmitters they release onto different downstream target neurons. The pattern of connectivity between the ChAT-expressing interneurons and their downstream targets also suggests a coherent function of releasing two neurotransmitters with opposite effects – GABA is released broadly onto inhibitory interneurons, shutting them down and preventing them from inhibiting further downstream neurons, while acetylcholine activates sparse subnetworks of downstream neurons. Future research will be needed to also understand if GABA and acetylcholine released from these neurons have additional modulatory effects, like controlling the degree of excitability of downstream neurons, or the extent to which they are able to change the strength of their synaptic connections.
Adam Granger is a postdoctoral fellow in the lab of Bernardo Sabatini.
Learn more in the original research article:
Cortical ChAT+ neurons co-transmit acetylcholine and GABA in a target- and brain-region-specific manner. Granger AJ, Wang W, Robertson K, El-Rifai M, Zanello AF, Bistrong K, Saunders A, Chow BW, Nuñez V, Turrero García M, Harwell CC, Gu C, Sabatini BL. Elife. 2020 Jul 2.
News Types: Community Stories
