By Carlos Manlio Díaz-García and Gary Yellen
Energy production is essential for sustaining brain activity. When we do a particular task, the neurons in specific parts of the brain become more active and need more energy. In seconds, these cells start consuming more fuel (usually glucose) to produce new energy, using both their mitochondria (organelles known as the “power plants” of the cell) and energy-producing reactions in the cytosol.
But how do cells coordinate these reactions, in order to keep their energy stores filled and to stay ready for further activity?
Calcium ion, a universal messenger in excitable tissues, is a candidate for coordinating metabolic responses in the cytosol and the mitochondria. It has well-known routes of entry into both compartments, it is energetically costly to extrude, and there are many calcium-signaling proteins that regulate metabolism or directly participate in reactions involved in energy production.
We investigated whether calcium influx into the cytosol contributes to the glycolytic response in stimulated neurons, which was unknown. Also, we explored the effect of calcium on the metabolic responses in the mitochondria, which has been controversial.
We used freshly-prepared slices of brain tissue to study metabolic responses of neurons within the mouse hippocampus, aided by artificially-engineered fluorescent biosensors of metabolism. Fluorescence microscopy then allowed us to measure both changes in intrinsically fluorescent (naturally-existing) molecules of metabolism and changes in the light output of the engineered fluorescent biosensors.
We found that calcium entry into mitochondria is required for strong activation of metabolism there, but in the cytosol, calcium functions more by increasing the demand for cellular energy rather than by signaling. Although eliminating the rise in cytosolic calcium eliminates most of the glycolytic response, increased energy demand from sodium entry and extrusion can restore part of it.
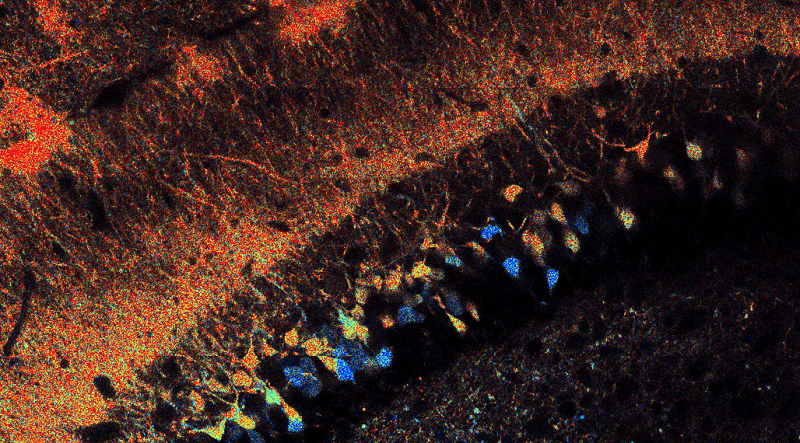
Hippocampal neurons expressing the metabolic sensor Peredox.
The cytosolic NADH/NAD+ ratio may differ among dentate granule cells in the mouse hippocampus, as revealed by the fluorescence lifetime of the biosensor Peredox (warmer colors indicate higher NADH levels)
The mechanisms by which calcium orchestrates neuronal metabolism are relevant to understanding how neurons cope with moment-to-moment changes in energy demand, and how neuronal metabolism adjusts to sustained periods of activity.
Coordination of energy production with brain activity is crucial to the proper functioning of the brain. Problems with this coordination may contribute to cognitive decline in neurodegenerative diseases. Inadequate resupply of energy can lead to pathology and death of brain cells, while particularly prompt resupply may exacerbate excessive neuronal activity in epilepsy. Understanding the control mechanisms for energy resupply may allow for better future treatment of these diseases by drugs or by changes in diet.
Carlos Manlio Díaz-García is a research fellow in the lab of Gary Yellen at Harvard Medical School
Gary Yellen is a Professor of Neurobiology at Harvard Medical School
Learn more in the original research article:
The distinct roles of calcium in rapid control of neuronal glycolysis and the tricarboxylic acid cycle
Díaz-García CM, Meyer DJ, Nathwani N, Rahman M, Martínez-François JR, Yellen G. Elife. 2021;10:e64821. Published 2021 Feb 8. doi:10.7554/eLife.64821
News Types: Community Stories
