by Seungwon (Sebastian) Choi
Each day we experience myriad somatosensory stimuli: hugs from loved ones, warm showers, a mosquito bite, sore muscles after a workout. Tactile, thermal, itch, and nociceptive stimuli are detected by peripheral sensory neuron terminals and end organs distributed throughout our body, propagated into the spinal cord, and then transmitted to the brain via ascending spinal pathways. Despite their central role as “somatosensory gateways” to the brain, very little is known about how spinal projection neurons (PNs) receive somatosensory signals from the periphery, and process and convey these signals to the brain to underlie perception of touch, temperature and pain. In our study, we used new mouse genetic tools in conjunction with anatomical, physiological and behavioral approaches to gain insights into the functional organization of ascending touch, thermal and pain pathways.
The key findings of our study are twofold. First, we found that two genetically defined PN 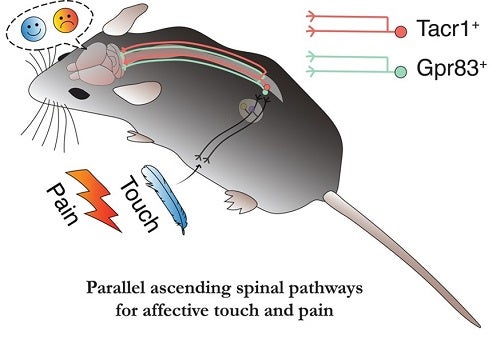 subtypes of the anterolateral pathway, a major ascending pain pathway, cooperate to convey nociceptive signals to the brain. The current view of ascending pain pathways emphasizes the involvement of TACR1(Substance P receptor)-expressing PNs in transmitting nociceptive signals from the spinal cord to the brain. However, therapeutic strategies in humans that target TACR1-expressing neurons, and TACR1 itself, to treat pain have been unsuccessful. This suggested to us the existence of additional, TACR1-negative spinal PNs that also convey pain signals to the brain. After conducting a screen of over 1300 transgenic GFP mouse lines, we identified a new PN subtype of the anterolateral pathway that expresses GPR83 (G protein-coupled receptor 83). We found that both GPR83- and TACR1-expressing PNs are responsive to noxious stimuli such as hot temperature and convey pain signals from the spinal cord to the lateral parabrachial nucleus (PBNL) of the pons, a brainstem structure that relays visceral and somatosensory information to forebrain regions. In addition, strong activation of either of the two spinoparabrachial PNs induces pain as evidenced by increased escape locomotion, autonomic response, and place aversion.
subtypes of the anterolateral pathway, a major ascending pain pathway, cooperate to convey nociceptive signals to the brain. The current view of ascending pain pathways emphasizes the involvement of TACR1(Substance P receptor)-expressing PNs in transmitting nociceptive signals from the spinal cord to the brain. However, therapeutic strategies in humans that target TACR1-expressing neurons, and TACR1 itself, to treat pain have been unsuccessful. This suggested to us the existence of additional, TACR1-negative spinal PNs that also convey pain signals to the brain. After conducting a screen of over 1300 transgenic GFP mouse lines, we identified a new PN subtype of the anterolateral pathway that expresses GPR83 (G protein-coupled receptor 83). We found that both GPR83- and TACR1-expressing PNs are responsive to noxious stimuli such as hot temperature and convey pain signals from the spinal cord to the lateral parabrachial nucleus (PBNL) of the pons, a brainstem structure that relays visceral and somatosensory information to forebrain regions. In addition, strong activation of either of the two spinoparabrachial PNs induces pain as evidenced by increased escape locomotion, autonomic response, and place aversion.
Second, we found that the GPR83-expressing PNs constitute an “affective touch” pathway that underlies pleasant or unpleasant feelings associated with tactile stimuli. The textbook view of the ascending touch pathway emphasizes the “discriminative touch” pathway, which conveys mechanosensory signals from the periphery to the somatosensory cortex via the spinal cord dorsal column, brainstem and thalamus. We found that touch signals are also conveyed by GPR83-expressing PNs to the PBNL–which in turn broadcasts these tactile signals to downstream brain regions, including the amygdala and hypothalamus, that influence social and emotional behaviors.
The GPR83-expressing PNs are highly sensitive to mechanical stimuli, and they transmit tactile information to both sides of the PBNL. In addition, axons of GPR83-expressing PNs representing different body parts such as forelimbs, hindlimbs, and body trunk regions innervate the same regions within the PBNL. The lack of laterality and somatotopy suggests that this pathway is not suitable for locating tactile stimuli – a discriminative aspect of touch. Instead, we found that weak activation of this pathway, which may reflect a gentle touch, is appetitive, while strong activation, which may reflect a harsh touch such as pinch, is aversive. Thus, the GPR83-expressing spinoparabrachial pathway underlies an affective aspect of touch perception in a scalable, stimulus intensity-dependent manner. It is noteworthy that human patients with anterolateral cordotomy often lose pleasant feelings associated with gentle touch while being able to locate where they are touched.
Overall, our study reveals two parallel ascending spinal pathways that convey affective touch and pain signals from the spinal cord to the brain. Our findings raise the intriguing possibility that “sensory gating” at the level of spinal output neurons is disrupted in neurological disorders associated with pain and affective touch. For example, since gentle touch can be perceived as painful or aversive in patients with neuropathic pain or autism spectrum disorders (ASDs), signals received or conveyed by the spinal PNs defined here may be altered. Future studies of the two spinal PN subtypes and the “druggable” GPCRs they express (TACR1 and GPR83) may reveal therapeutic approaches for treating sensory over-reactivity in neuropathic pain states and ASDs.
Seungwon (Sebastian) Choi is a postdoctoral fellow in David Ginty’s lab, in the Department of Neurobiology at Harvard Medical School. The Ginty lab’s research was performed in collaboration with labs at the University of Pittsburgh.
This story will also appear in the HMS Neurobiology newsletter, The Action Potential
Learn more in the original research article:
Parallel ascending spinal pathways for affective touch and pain.
Choi S, Hachisuka J, Brett MA, Magee AR, Omori Y, Iqbal NU, Zhang D, DeLisle MM, Wolfson RL, Bai L, Santiago C, Gong S, Goulding M, Heintz N, Koerber HR, Ross SE, Ginty DD. Nature. 2020 Oct 28. doi: 10.1038/s41586-020-2860-1. Online ahead of print.
News Types: Community Stories
