By Xiangsunze Zeng & Clifford Woolf
The spinal cord projects sensory signals to the brain that trigger the sensation of touch, temperature and pain. From the dorsal horn, a set of specialized output neurons carry pain-related information into the brain through the anterolateral tract. We wanted to know how the coding of these spinal signals changes when pain goes from a short-term acute state to a chronic, neuropathic state. To address this, we used an advanced technique called longitudinal calcium imaging in mice – essentially a way to watch, through a spinal window, how nerve cells ‘light up’ in response to stimuli over time. We monitored the activity of the same spinal neurons repeatedly over months.
Under normal conditions, the spinal projection neurons in the superficial dorsal horn of the spinal cord fall into several distinct groups: Some respond exclusively to cooling of the skin, others to warming, and a third group are only activated in response to intense, painful heat or mechanical stimuli. These stable, highly selective pathways ensure that different types of sensations—cooling, warmth, and pain—remain distinct.
We asked how this picture changes during pain conditions. To study acute pain, we applied capsaicin—the active component of chili peppers, which binds TRPV1 receptors to trigger a burning sensation—to skin. This method temporarily sensitizes TRPV1 expressing sensory neurons and causes a brief, burning pain lasting for tens of minutes. After this treatment, the spinal neurons become more broadly responsive: cells that typically react only to strong, noxious stimuli begin to fire in response to low-intensity, innocuous heat or pressure. In other words, the nervous system’s threshold is turned down. Notably, this change is short-lived. As the effects of capsaicin wear off, the spinal neuronal responses return to their original, narrowly tuned state —like a temporary glitch that fixes itself.
To model chronic neuropathic pain, we injured peripheral nerves — an established method to mimic long-term nerve damage in humans. In this model, the sensory landscape changes profoundly. Many spinal neurons that previously responded to gentle warmth become suppressed. At the same time, a new group of neurons emerge: a population that had been quite silent under healthy conditions. These “SILENT” neurons now begin firing in response to intense stimuli such as noxious heat—signals they had ignored before. Unlike the transient sensitization seen with capsaicin, this switch is persistent: these neurons remain permanently altered long after the injury.
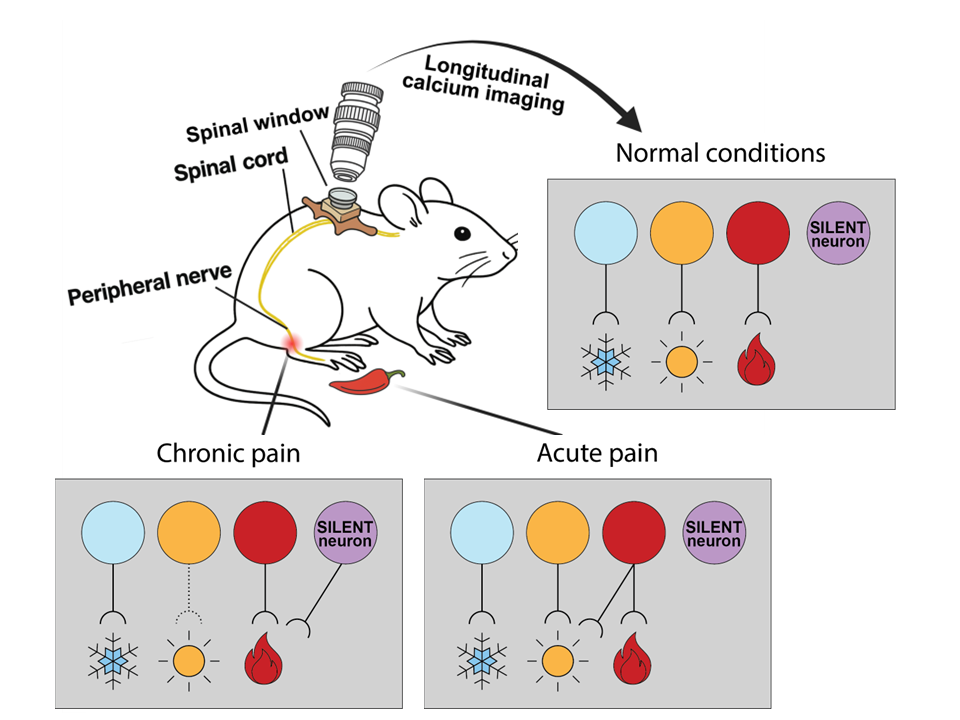
• Normal conditions: Distinct spinal neuron groups carry signals for cold, warmth, and painful heat.
• Acute pain (capsaicin): Pain neurons become temporarily more sensitive, responding to low-level warm stimuli but then return to their high-threshold state soon after.
• Chronic pain (peripheral nerve injury): Responses to gentle warmth are diminished, while previously silent neurons (purple) become persistently active in response to high-intensity signals.
Understanding how pain pathways are reorganized in the spinal cord in the setting of persistent pain brings us closer to the possibility that we can selectively block chronic pain with precision, and without disrupting the body’s essential, protective responses to real danger. Our findings reveal a differential modification of the way that spinal neurons adapt across different pain states (see Figure). By identifying which spinal neurons carry pain-triggering signals—and how their tuning changes in chronic pain—we move closer to developing novel, targeted treatments. For example, drugs or gene therapies that selectively inhibit the maladaptive “SILENT” neurons may prevent harmless stimuli from being perceived as painful without disrupting normal temperature sensation. In other words, we may be able to quiet overactive pathological ‘messengers’ without cutting the entire phone line.
Xiangsunze Zeng is a post-doctoral research fellow in the lab of Clifford Woolf in the FM Kirby Neurobiology Center at Boston Children’s Hospital and Harvard Medical School.
Clifford Woolf is a Professor of Neurobiology and Neurology at Harvard Medical School and the Director of the F.M. Kirby Neurobiology Center at Boston Children’s Hospital.
Learn more in the original research paper:
Differential modification of ascending spinal outputs in acute and chronic pain states
Yarmolinsky DA, Zeng X, MacKinnon-Booth N, Greene CA, Kim C, Cheng YT, Lenfers Turnes B, Woolf CJ. Neuron. 2025 Apr 16;113(8):1223-1239.e5.PMC12005971.
News Types: Community Stories
