By Krishna K. Narayanan, Matthew L Baum, Matthew Johnson, and Beth Stevens
Neurons create complex connections with each other called synapses to build circuits, transmit signals, and communicate. These connections are overabundant in early life to allow for plasticity and learning but, as brains learn and grow into adulthood, phagocytic cells in the brain called microglia physically engulf the excess or unused synapses to create long-term-stability in brain circuits – a process known as synaptic pruning. Signaling proteins called complement proteins that are present on neuronal surfaces guide the microglia to engulf certain synapses. Synaptic pruning can go awry during development due to many known and unknown risk factors and may contribute to neurodevelopmental and psychiatric illnesses. Most notably, two genetically implicated risk factors for schizophrenia are the complement component C4, and a little-studied protein called CUB and Sushi Multiple Domains 1 (CSMD1), which is highly abundant in the brain and possesses structural similarities to proteins that regulate complement elsewhere in the body. Therefore, we investigated whether CSMD1 interacts with complement proteins in the brain and thereby affects microglia-mediated synaptic pruning.
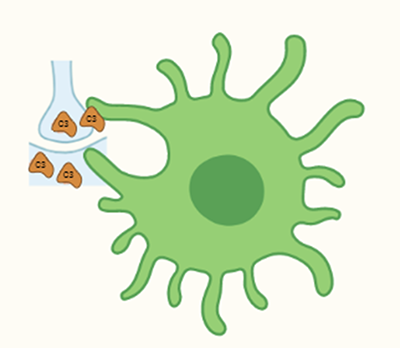
Schematic of a microglial cell engulfing a synapse (image detail from the graphic abstract of the research paper, Baum et. al., 2024, created with biorender.com)
Our lab found that CSMD1 is indeed present at synapses and interacts with complement components C1q and C4 in the mouse brain. When examining mice that lacked CSMD1, we discovered increased complement signals at synapses, and fewer synapses, in a circuit – the retinogeniculate system – well-known to be developmentally sculpted by microglia-mediated pruning, suggesting that CSMD1 is important for regulating synapse numbers. Similarly, when we deposited complement signals onto human stem cell-derived neurons grown in vitro, neurons that lacked CSMD1 ended up with more surface-bound complement than control neurons. Finally, we found that cultured mouse microglia favored engulfing synapses isolated from CSMD1 knockout mouse brains relative to synapses from their wild-type littermates. These results support a model in which CSMD1 opposes the deposition of pro-engulfment complement signals on synapses in both mice and humans, and suggest that reduced CSMD1 expression could contribute to risk for schizophrenia via enhanced synaptic pruning.
Taken together with previous publications from our lab and others showing that increased complement C4 expression has similar effects, our current work constitutes an exciting convergence of two independent common genetic risk factors for schizophrenia onto the same molecular and cellular mechanism, increasing support for the synaptic pruning hypothesis of schizophrenia and for this pathway and mechanism as a potential target for therapeutic development.
Krishna K. Narayanan, Ph.D. is a project manager in Beth Stevens lab and has an interest in public communication of science.
Matthew L Baum, MD, PhD, DPhil is a psychiatrist, neurobiologist, and neuroethicist. He completed his PhD in Beth Stevens Lab and recently transitioned to faculty at Brigham and Women’s Hospital.
Matthew Johnson, PhD is a developmental neurobiologist and a group leader in the Stanley Center for Psychiatric Research of the Broad Institute of MIT and Harvard.
Beth Stevens, PhD is a developmental neurobiologist and the leader of the Stevens Lab at Boston Children’s Hospital and the Stanley Center for Psychiatric Research of the Broad Institute of MIT and Harvard
Learn more in the original research article:
CSMD1 regulates brain complement activity and circuit development.
Baum ML, Wilton DK, Fox RG, Carey A, Hsu YH, Hu R, Jäntti HJ, Fahey JB, Muthukumar AK, Salla N, Crotty W, Scott-Hewitt N, Bien E, Sabatini DA, Lanser TB, Frouin A, Gergits F, Håvik B, Gialeli C, Nacu E, Lage K, Blom AM, Eggan K, McCarroll SA, Johnson MB, Stevens B. Brain Behav Immun. 2024 Jul;119:317-332.
News Types: Community Stories
