By Yuqin Yin and Larry Benowitz
Nerve pathways in the mature CNS cannot regenerate if injured, and consequently, victims of stroke, spinal cord injury or other types of CNS damage commonly sustain lifelong disabilities. Using the optic nerve as an example of a CNS pathway that normally fails to regenerate when injured, our lab and others have previously identified strategies to enable some retinal ganglion cells (RGCs, the projection neurons of the eye) to regenerate axons through the optic nerve and back to the brain in mice. However, the incompleteness of this regeneration underlines the need for a better understanding of the factors that enable or suppress regeneration.
Our new study set out to discover “master switches” that enable RGCs to change from a quiescent, non-growing state to a regenerative state. To do this, we studied RGCs exposed to either a strongly pro-regenerative vs. a control treatment following optic nerve injury. We separated the RGCs from the other cells of the retina by fluorescence-activated cell sorting and investigated their gene expression profiles using RNA (whole transcriptome) sequencing. The Geschwind group at UCLA then used bioinformatics to predict the transcription factors that regulate expression of genes that are differentially expressed in the regenerative state.
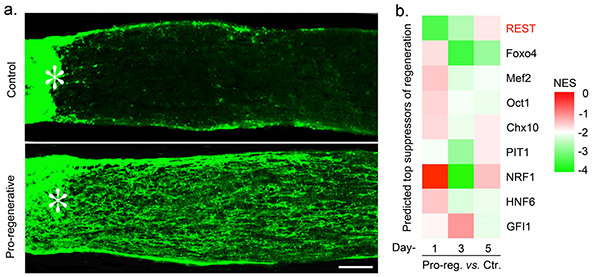
a. Axons (nerve fibers) regrowing through the injured optic nerve are visualized by green fluorescent staining. Asterisks denote the injury site. Whereas few if any axons normally regenerate when the optic nerve is injured (top), a treatment described in the paper enables retinal ganglion cells (RGCs), the projection neurons of the retina, to regenerate axons.
b. Schematic diagram shows a bioinformatic analysis of transcription factors that bind upstream to the coding regions of genes that are differentially expressed in the regenerative state. These transcription factors are listed on the right. The differences in colors across conditions indicates the predicted differences in the involvement of these transcription factors in regulating the regenerative state. The prediction that REST (NRSF) is a primary suppressor of RGCs’ ability to regenerate axons was borne out in experiments showing that deleting the REST gene or expressing a non-functional form of REST in RGCs was sufficient to promote considerable regeneration. The same study found that deletion of REST in cortical neurons enables axon regeneration in the injured spinal cord.
Data analysis predicted that REST (NRSF), a transcription factor known to repress the ability of non-neuronal cells to express neuron-specific genes, also represses the expression of genes required for axon regeneration in mature RGCs. We tested this prediction using mice in which we either deleted the gene encoding REST in RGCs or expressed a mutant form of REST that binds to target genes but fails to enlist essential co-factors. Both treatments induced considerable optic nerve regeneration.
Complementary studies by the Sofroniew group (UCLA) showed that deleting REST in neurons of the motor cortex promotes axon regeneration in the corticospinal tract, a pathway that is essential for voluntary movements that is notoriously resistant to pro-regenerative treatments. Thus, REST appears to function widely to repress axon regeneration in the mature CNS, and its deletion is sufficient to promote appreciable levels of axon regeneration in the optic nerve, spinal cord, and perhaps other CNS pathways. When combining it with other effective treatments, we expect to see much stronger regeneration. The final goals of this research are to attain functional recovery of injured nerve pathways ultimately leading to recovery of vision and/or improving outcome for paralyzed patients.
Yuqin Yin is an Instructor in Neurosurgery at Boston Children’s Hospital and HMS.
Larry Benowitz holds the Neurosurgical Innovation and Research Endowed Chair at BCH and is a Professor of Neurosurgery and Ophthalmology at HMS.
Learn more in the original research article:
Transcription factor network analysis identifies REST/NRSF as an intrinsic regulator of CNS regeneration in mice
Cheng Y, Yin Y, Zhang A, Bernstein AM, Kawaguchi R, Gao K, Potter K, Gilbert HY, Ao Y, Ou J, Fricano-Kugler CJ, Goldberg JL, He Z, Woolf CJ, Sofroniew MV, Benowitz LI, Geschwind DH. Nat Commun. 2022 Jul 29;13(1):4418. doi: 10.1038/s41467-022-31960-7. PMID: 35906210
News Types: Community Stories
