By Will Renthal and Ivan Tochitsky
Peripheral sensory neurons can regenerate after a nerve injury and restore normal sensation. Spinal cord neurons cannot, however, regenerate after injury, leading to permanent sensory loss and paralysis. Why are only some neuronal types able to regenerate after axonal injury?
Peripheral sensory neurons transmit signals from nerve endings in the skin, muscle, joints and viscera to the spinal cord and brain, carrying information about the environment such as temperature and pressure. Peripheral nerve terminals are fragile and easily damaged, so axonal regeneration is critical for maintaining function after tissue injury.
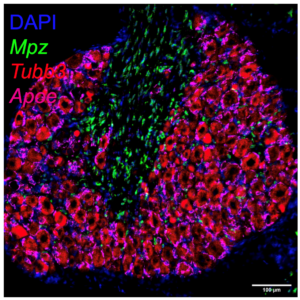
Dorsal root ganglion cells stained for Mpz (Schwann cells), Tubb3 (Neurons), Apoe (satellite glia).
To understand the peripheral neuronal respond to injury, we characterized the changes in gen eexpression that take place in mouse peripheral sensory neurons after traumatic axonal injury using single-nucleus RNA-sequencing. We found that injured sensory neurons exhibit an intrinsic capacity to reprogram their gene expression patterns (see www.painseq.com), transforming them from cells highly specialized for sensing and transmitting specific sensations, to cells focused on axon regeneration and reinnervation of their normal targets in the skin and muscle. We identified key factors that help drive this transcriptional reprogramming after injury, which may hold clues for how to adapt this regenerative capacity to central nervous system neurons. Several of these transcription factors resemble those that are used to convert mature cells to induced pluripotent stem cells, suggesting that the physiological reprogramming that occurs after axonal injury might involve analogous mechanisms.
Future studies will be aimed at further understanding the epigenomic mechanisms underlying injury-induced transcriptional reprogramming of peripheral sensory neurons and the ways of translating this information to a mechanistic understanding of neurological disorders such as spinal cord injury, neurodegeneration, and chronic pain.
Will Renthal is Assistant Professor of Neurology at Harvard Medical School and the Director of Research at the John R. Graham Headache Center at Brigham and Women’s Hospital. He has launched a new lab focused on the genetics and epigenomics of headache and pain. He recently completed a fellowship with Michael Greenberg.
Ivan Tochitsky is a scientist at Regeneron. He was previously a postdoctoral fellow in the lab of Clifford Woolf.
Learn more in the original research article:
Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, Kawaguchi R, Geschwind DH, Woolf CJ.Neuron. 2020 Aug 14:S0896-6273(20)30570-5. doi: 10.1016/j.neuron.2020.07.026.
News Types: Community Stories
