By Lili Xie and Larry Benowitz
The optic nerve conveys visual information from the eye to the brain. Unfortunately, like other pathways in the central nervous system (CNS), the optic nerve cannot regenerate when injured, resulting in lifelong blindness for victims of traumatic or ischemic damage to this pathway or in degenerative diseases such as glaucoma. Over the past 20 years or so, our lab and others have identified ways to stimulate retinal ganglion cells (RGCs), the nerve cells in the eye that convey visual signals to the brain, to begin regenerating injured axons back the brain. However, the extent of regeneration and visual recovery achieved to date remain modest, pointing to the need to identify better ways to stimulate the regrowth of connections from the eye and the brain.
Ciliary neurotrophic factor (CNTF) is a leading therapeutic candidate for glaucoma and other ocular diseases and is often used experimentally to promote axon regeneration after optic nerve injury. Elevating CNTF levels using a viral vector to carry the gene into RGCs (gene therapy) leads to considerable axon regeneration and is neuroprotective for RGCs. Surprisingly, however, intraocular injections of the CNTF protein has little effect. We discovered that CNTF gene therapy causes a substantial inflammatory reaction in the eye that results in elevated expression of the chemokine CCL5 in immune cells that enter the eye and in glial cells of the retina. Through experiments that either reduce the effects of CCL5 (genetic deletion of its receptor in RGCs, deletion of CCL5 in immune cells, use of a pharmacological blocker) or that increase CCL5 levels, we showed that CCL5 in fact mediates most of the beneficial effects of CNTF gene therapy.
Thus, this study identifies CCL5 as a potent, previously unknown agent for optic nerve regeneration. Our results also raise general questions about interpreting the results of gene therapy studies.
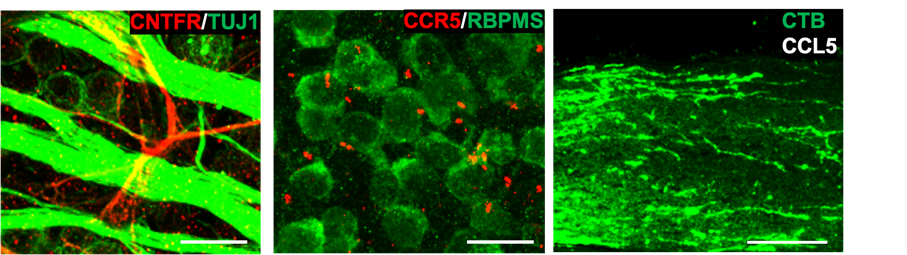
(a) Portion of the mouse retina showing that the CNTFR (red), the receptor through which CNTF acts, is not present in retinal ganglion cells (RGCs) or their axons (green). Other experiments show that the cells that express the receptor are astrocytes. This finding suggests that the effects of CNTF gene therapy on RGC survival and optic nerve regeneration do not involve CNTF acting directly on RGCs, and instead are likely to involve one or more other factors. Scale bar, 10 µm. (b) Mouse retina immunostained to visualize the chemokine receptor CCR5 (red) and RGCs (anti-RBPMS) (green) (Scale bar, 10 μm). CCR5 is localized in the primary cilia of RGCs. (c) Section of the mouse optic nerve showing regenerating axons (green) after injecting CCL5 into the eye following optic nerve damage (Scale bar, 150 µm).
Lili Xie is a PhD student in the lab of Larry Benowitz.
Larry Benowitz is a Professor of Neurosurgery and Ophthalmology at Harvard Medical School and a Neurosurgical Innovation and Research Endowed Professor at Boston Children’s Hospital.
Learn more in the original research article:
Chemokine CCL5 promotes robust optic nerve regeneration and mediates many of the effects of CNTF gene therapy.
Xie L, Yin Y, Benowitz L. Proc Natl Acad Sci U S A. 2021 Mar 2;118(9):e2017282118. doi: 10.1073/pnas.2017282118. PMID: 33627402; PMCID: PMC7936361.
News Types: Community Stories
