By Neil Dani and Maria Lehtinen
Best known for producing cerebrospinal fluid, the choroid plexus (ChP) is a brain structure emerging as a complex, multifunctional network of diverse cell types that does much more — participating in brain development, homeostasis, and defense against injury and disease. The ChP regulates fluid and electrolyte balance, serves as a portal for immune cell entry, secretes critical growth factors, and upregulates host defense gene programs in aging. The ChP is also a potential site of SARS-CoV-2 virus infection in the central nervous system. Further, the ChP holds great promise as a therapeutic target by serving as a delivery system of genetically engineered proteins to the brain via the CSF. However, incomplete characterization of its cellular and molecular properties has hampered the scientific community’s efforts to harness ChP biology for therapeutic intervention. In our recent study, done in collaboration with Rebecca Herbst, Naomi Habib and Aviv Regev at the Broad Institute, we coupled single cell/nucleus transcriptomics and imaging approaches to generate the first comprehensive cellular and spatial atlas of the ChP across ventricles (CSF-filled brain regions) of the developing, adult and aging mouse brain.
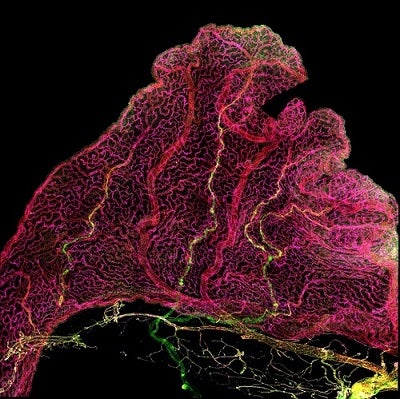
Immunofluorescence imaging of choroid plexus reveals diversity across endothelial cells (red). Yellow blood vessels mark Claudin-5 expressing arteries that enter and extend into the choroid plexus from the brain, while magenta-colored vessels mark the network of capillaries.
Transcriptomic profiling revealed ChP topography and age-dependent gene signatures of epithelial, endothelial, mesenchymal, neuronal, glial and immune cells. For example, in mouse embryos (embryonic day E16.5) insulin expression was enriched in epithelial cells of the third ventricle–which may function as a source of this neuroendocrine signal in the developing brain. We also found a progenitor cell niche in the fourth ventricle of the hindbrain that gives rise to ChP epithelial cells and neurons that go on populate the cerebellum, a nearby brain structure involved in coordinating voluntary movement. In aging, multiple cell types upregulated neuro-immune functions, including macrophages that upregulated inflammatory gene expression and aging epithelial cells that upregulated host-defense programs. In contrast, some neuro-immune functions were stable across ventricles and ages, as ChP pericytes and some epithelial cells stably expressed the ACE2 receptors that are co-opted by the SARS-CoV-2 for cell entry.
Overall, this new ChP atlas provides great insight into the cellular and molecular properties of the ChP blood-CSF barrier that will guide further basic and therapeutic neuroscience research for years to come.
Neil Dani is an Instructor in pathology and postdoctoral fellow in the Department of Pathology at Boston Children’s Hospital. Maria Lehtinen is Associate Professor in Pathology at Boston Children’s Hospital and Harvard Medical School.
Learn more in the original research article:
A cellular and spatial map of the choroid plexus across brain ventricles and ages. Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, Head JP, Cui J, Shipley FB, Jang A, Dionne D, Nguyen L, Rodman C, Riesenfeld SJ, Prochazka J, Prochazkova M, Sedlacek R, Zhang F, Bryja V, Rozenblatt-Rosen O, Habib N, Regev A, Lehtinen MK. Cell. 2021 Apr 27:S0092-8674(21)00438-4. doi: 10.1016/j.cell.2021.04.003. Epub ahead of print. PMID: 33932339.
News Types: Community Stories
