By Chuck Phillips
From pressure to pianissimo
Music, the voice of a loved one, the din of chirping crickets on a summer evening, and the myriad sounds we take for granted in a day, in their most elementary form are all a kind of mechanical energy — waves of changing air pressure. Because the brain detects and understands the outside world by electrical signaling, the ear needs a way of transforming, or ‘transducing’, that mechanical energy into electricity so we can hear. For that, a molecular machine is used — an ion channel — a membrane-embedded protein that produces an electric current by passing ions through its pore when it’s opened by sound-generated forces. The process, called ‘auditory mechanotransduction’, takes place in the cochlea and is mediated by the ion channel TMC1 located at the tips of stereocilia, hair-like structures on the apical surface of hair cells arranged in rows of increasing height. When the stereocilia are deflected toward the tallest row by sound-induced pressure waves, TMC1 opens and conducts potassium through its pore, changing the cell’s electrical properties in a way that leads to neuronal signaling.
A molecular gate
How TMC1 moves as it opens in response to force, or ‘gates’, has remained an important question since its implication in auditory mechanotransduction seven years ago. Genetic mutations in TMC1 that effect channel gating compromise auditory mechanotransduction and could lead to hearing defects, including deafness.
TMC1 exists in an ion channel complex with several other proteins in the shorter rows of stereocilia and is connected to the taller rows by a protein tether called the ‘tip-link’: It is through this connection, and the tension derived in the tip-link upon hair-bundle deflection, that force is conveyed to the TMC1 ion channel complex. How the tip-link physically attaches to the complex has remained a mystery and makes the TMC1 gating question — how does TMC1 open in response to force — a difficult one to answer. We asked, “how does the tip-link physically interact with TMC1?” to better understand how TMC1 might open in response to force.
Two theories
There are two general mechanisms by which TMC1 could open in response to tip-link mediated force. In the “force from lipid model”, membrane tension directly opens the channel: The tip-link — which has a transmembrane domain and like TMC1, is embedded in the membrane — pulls on the membrane near the channel and changes the free-energy landscape in the membrane near TMC1 to favor its open state. Biophysical models for this mechanism predict that physiologically plausible membrane tension might be generated. In the “tether model”, the tip-link binds directly to TMC1, or to one of its complex members which binds to TMC1, and pries the channel open through direct protein-protein interactions.
A deep dive in
Our lab recently used biochemical techniques to show that TMC1 and the tip-link directly interact and that the interaction takes place extracellularly. Specifically, we found the extracellular EC11 domain of PCDH15 — the protein subunit of the tip-link closest to the channel complex — binds to an extracellular region of TMC1 we call the ‘1-2 loop’. This loop also binds near TMC1’s ion permeation pathway in our ‘closed-like’ structure prediction and moves away from the pore in our ‘open-like’ TMC1 structure, resembling a gate that opens and closes over the ion pathway.
An AlphaFold3 structure of TMC1 and PCDH15 together predicts that the primary binding interface is between EC11 and a short helical segment in the 1-2 loop, a result consistent with our biochemistry data. This short helical segment is highly conserved in TMC homologues expressed in hair cells (TMC1-TMC3) and is not conserved in homologues not expressed in hair cells (TMC4-TMC8), suggesting it has a hearing-specific function. Also, mutations in the 1-2 loop change the gating behavior of TMC1 as assessed by whole cell patch-clamp current recordings in response to hair-bundle deflections in mouse hair cells.
A tentative new gating model
Based on these results, it is tempting to paint a picture of TMC1 gating by which the tip-link pulls on the 1-2 loop of TMC1 to pry the pore open upon sound-induced, tip-link-mediated force. However, further studies will be necessary to validate this model, for example, by introducing point mutations in TMC1 or PCDH15 that both interrupt their binding and alter TMC1 gating. Atomic resolution images of the fully assembled TMC1 complex in hair cells — ideally, one under tip-link tension and another not — would also provide insight into the gating mechanism of TMC1.
Overall, our work considers a novel molecular mechanism by which the ear — in a way like beetles converting the strawberries they eat into life-giving energy — converts the mechanical energy of sound into the sublime sensation of hearing.
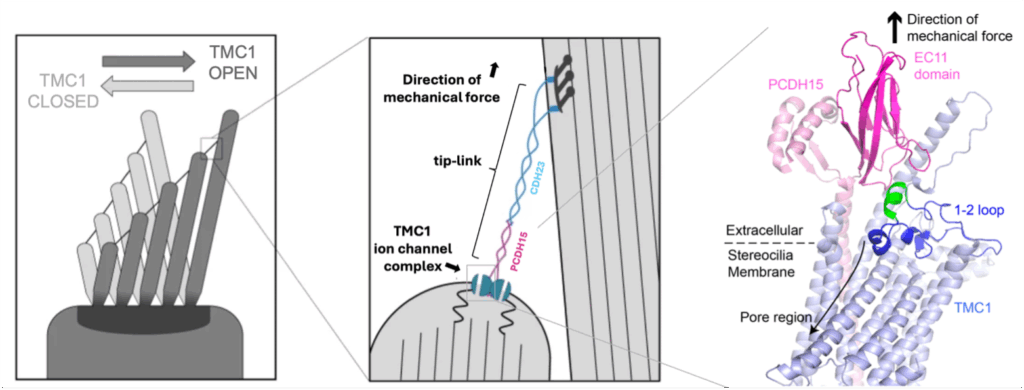
Left, cartoon of the apical region of a hair cell. A tension force in generated in the tip-link connecting the stereocilia when they are deflected toward the tallest row. This force opens the TMC1 ion channel which then conducts an electric current across the membrane. Middle, zoomed in view of the tip-link and auditory mechanotransduction ion channel complex from boxed area on left. It was previously unclear if, and if so, how the tip-link interacts with TMC1 in the ion channel complex. Right, AlphaFold3 structure prediction of PCDH15 (pink) and TMC1(blue). The EC11 domain of PDCH15 (dark pink) binds to the 1-2 loop of TMC1 (dark blue and green). The short helical segment of TMC1’s 1-2 loop (green) interacts directly with PCDH15 and is highly conserved in TMC homologues expressed in hair cells and not conserved in homologues not expressed in hair cells. It is tempting to speculate that the tip-link pulls the 1-2 loop away from the pore region to allow for ion conduction.
News Types: Community Stories
