By Connie Cepko
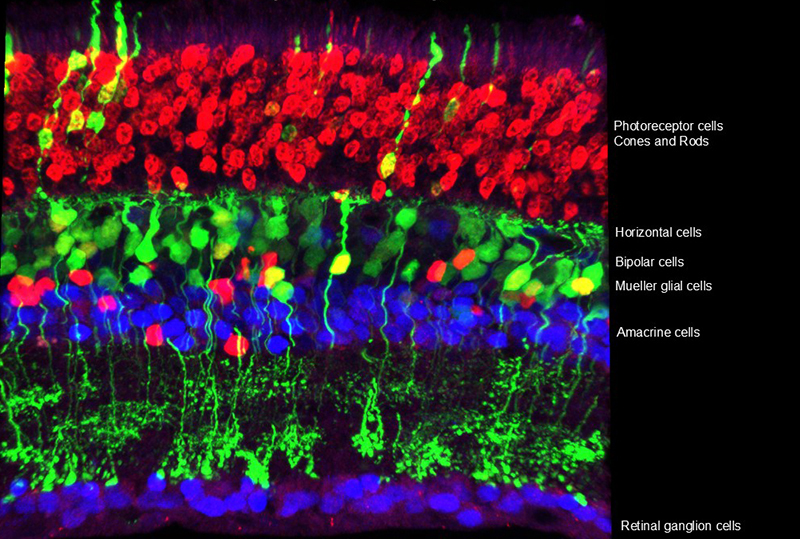
A Cross Section of the Mouse Retina
The retina comprises many types of cells. This diversity creates local circuits that transform the information of light wavelength and intensity into features that are useful to an organism. In this way, it is not simply a camera, but is a parallel processing computer, calculating such features as the direction of motion, edges, and changes in light intensity. Shown here is a cross section from a mouse retina, which is very similar to that of humans. Several cell types are labelled on the image. Some cell types have many sub-types, with the current tally of types and sub-types now greater than 100.
Image from Sui Wang, lab of Connie Cepko.
We humans are very visual creatures. We rely on vision for more than reading the internet. Vision is a critical component of how we communicate with each other, being very tuned to people’s expressions and body language. Loss of vision is one of the most feared medical and lifestyle conditions. Our laboratory is trying to do something about that.
We have been studying the retina, the part of the eye that receives and processes light information. The retina is much more than a camera. It is more akin to a parallel processing computer. In the eye, local circuits transform incoming light information into patterns of color, directions of motion, and so much more.
All of this starts with the photoreceptors—called rods and cones—which receive and process light. Our rods are the most abundant type of photoreceptor and give us our night vision. Cones operate in the brighter light intensities during the day and provide us color and high acuity vision.
There are many genetic diseases that affect rods and cones, leading to poor vision or blindness. There are also disease genes that affect cells that support the function of rods and cones. Prominent among these are the retinal pigmented epithelial (RPE) cells. We have been studying how these disease genes affect the rods, cones, and RPE cells, and cause loss of vision. As we gain greater understanding, we design therapies that address these problems.
We have learned that, during the progression of disease, the eye suffers from oxidative damage, inflammation, and metabolic problems. To address these problems, we create viral vectors, based upon a non-pathologic virus, adeno-associated virus (AAV). We equip these AAVs with genes that combat each problem, or several problems at once. To test these treatments, we use mice or rats that have mutations that create the same genetic problems inherited by some people. In addition, we can mimic some non-genetic insults, as in age-related macular degeneration, the leading cause of blindness in people over 75 years of age. We deliver these AAV vectors to the eye in animal models and then study how our treatment affects the animal’s vision and eye structure.
To date, we have seven treatments that succeed in prolonging vision in mouse and rat models of disease. We hope to someday deliver these AAVs to people with a range of different genetic or environmental problems, rather than target only one particular gene mutation. This hopefully will allow us to help a greater number of people with eye diseases.
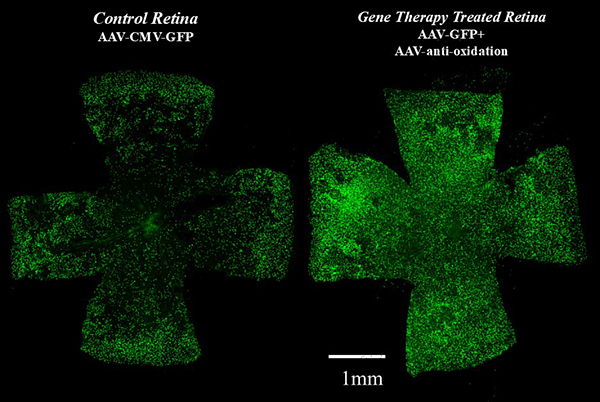
Gene Therapy to Combat Oxidation Can Prolong Cone Survival
A mouse with a blinding disorder, retinitis pigmentosa (RP), was treated with a virus that fights oxidative damage. During RP, oxidative damage can lead to the death of cone photoreceptors, the cell type that is needed for color and high acuity vision. The therapeutic virus was delivered to the eye of young mice. Several weeks later, the survival of cones, as well as vision, were measured. Shown here on the left is the retina of an animal that received treatment with an irrelevant virus, and on the right is a retina that received the therapeutic virus. Each green dot represents a cone. There are more cones, and better vision, following treatment with the therapeutic virus.
Image from Xiong W et al. 2015. J Clin Invest. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage.
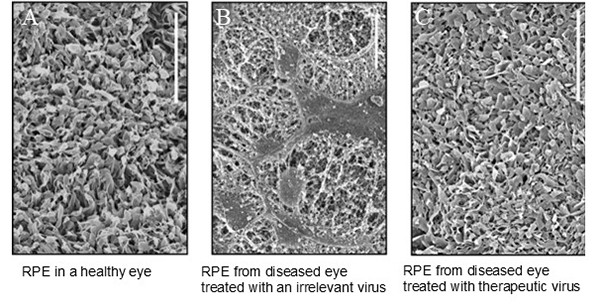
Gene Therapy Can Preserve the Retinal Pigment Epithelium in Retinitis Pigmentosa
A mouse with a blinding disorder, retinitis pigmentosa (RP), was treated with gene therapy. The retinal pigmented epithelial cells (RPE), which are necessary for vision, degenerate in RP. The RPE uses microvilli, extensions of the surface of the RPE (seen here using scanning electron microscopy), to surround the photoreceptors and provide support to them (left panel). In the RP disease, the microvilli degenerate and eventually the RPE cells die (middle panel). With the addition of a therapeutic virus, AAV-Best1-Nrf2, the RPE microvilli are preserved (right panel).
Image shows data from David Wu and Maryna Ivanchenko. Wu DM et al. 2021. JCI Insight. Nrf2 overexpression rescues the RPE in mouse models of retinitis pigmentosa.
Connie Cepko is Professor of Genetics and Ophthalmology at Harvard Medical School and an HHMI Investigator.
To learn the latest in this line of research, check out this recent article from the Cepko Lab:
RPE-specific MCT2 expression promotes cone survival in models of retinitis pigmentosa.
Chandler LC, Gardner A, Cepko CL. Proc Natl Acad Sci U S A. 2025 Apr 8;122(14):e2421978122. doi: 10.1073/pnas.2421978122. Epub 2025 Apr 3
News Types: Community Stories
