By Alexandra Vaccaro and Yosef Kaplan Dor
Why do we spend a third of our lives sleeping? Is sleep essential for survival? In the 1980s, Allan Rechtschaffen and colleagues reported that long-term sleep deprivation in rats is lethal. Similar observations were made in other model organisms, including fruit flies, but the underlying mechanism remained a mystery.
It has been assumed that death during sleep loss results directly from impaired brain function, but evidence of significant cell injury was never found in sleep-deprived brains. At the same time, many diseases are associated with poor sleep – diabetes, cardiovascular disease, even cancer. This implies that other parts of the body are affected when sleep is restricted. To understand what kills sleep-deprived animals, we systematically examined fruit flies for signs of cellular damage throughout the body during long-term sleep deprivation.
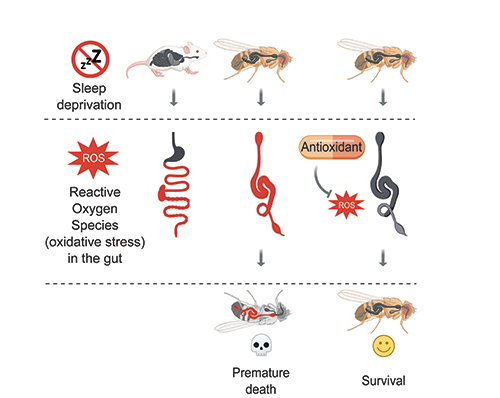
Sleep deprivation induces ROS accumulation and oxidative stress in the gut of mice (left) and flies (middle). In flies, ROS accumulation leads to premature death (middle, bottom). Death of sleep-deprived flies can be prevented through neutralization of intestinal ROS (right).
Artist/image source: Rogulja lab. Created with BioRender.com.
We found high levels of reactive oxygen species, ROS, specifically in the gut. At physiological levels, ROS have many important functions, but when their levels become abnormally high, they oxidize and damage DNA, lipids, and proteins, which can eventually trigger cell death. Indeed, ROS accumulation caused widespread oxidative damage and cell death in the gut of sleep-deprived flies. In addition to flies, we examined sleep-deprived mice – their small and large intestines also exhibit specific ROS accumulation and subsequent oxidative damage, suggesting that we have uncovered a conserved phenomenon.
Is intestinal ROS accumulation the cause of death in sleep-deprived animals? To answer this question, we asked if preventing ROS accumulation in the gut of sleep-deprived flies prolongs their survival. Importantly, clearing ROS from the gut using oral antioxidants or gut-targeted overexpression of antioxidant enzymes allowed normal lifespan despite almost complete lack of sleep. Contrary to the gut-targeted survival rescue, overexpressing antioxidant enzymes in the nervous system extended survival only by a few days. These experiments indicate that severe sleep-deprivation kills flies primarily through ROS accumulation in the gut.
Our work points to the gut as a major site of impact when sleep is severely suppressed. Similar oxidative processes may take place in the gut during chronic lack of sleep in humans, a common occurrence in the modern society. Our observations could potentially explain the link between insufficient sleep and some of the pathologies (including gastro-intestinal diseases) associated with sleep disturbances. We are now investigating how sleep deprivation causes ROS accumulation preferentially in the gut, and what the consequences of this increase in ROS levels are. We hope that this will help us better understand what the physiological basis for sleep need is, with the ultimate goal of developing strategies to ameliorate the negative impact of inadequate sleep.
Alexandra Vaccaro and Yosef Kaplan Dor are postdocs in the lab of Dragana Rogulja at Harvard Medical School.
This story will also appear in the HMS Neurobiology Department newsletter, The Action Potential.
Learn more in the original article:
Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Alexandra Vaccaro, Yosef Kaplan Dor, Keishi Nambara, Elizabeth A. Pollina, Cindy Lin, Michael E. Greenberg, Dragana Rogulja. Cell, 2020 June 11.
News Types: Community Stories
