By Evelyn Avilés
Polarization of neural circuits is critical for the proper flow of information and relies on the orderly arrangement of neurons, their processes, and their synapses. This polarity is apparent at the level of the tissue in the retina. For example, retinal amacrine cells sit at the bottom of the inner nuclear layer and extend a single dendritic arbor into the inner plexiform layer (IPL), thereby modulating signals as they travel from photoreceptors in the outer retina to retinal ganglion cells in the inner retina. Developmentally, this pattern emerges through a series of sequential cellular processes, such that neuronal precursors migrate to the right place, orient their processes in a certain direction, and then form synapses in a specific location. We are trying to understand how are these events are coordinated across the retina and across developmental time.
Previous work from our lab showed that the cadherin Fat3 – a member of the tissue polarity family of proteins – is asymmetrically localized to amacrine cell neurites pointed towards the IPL. In the absence of Fat3, amacrine cell somas are misplaced and their trailing neurites do not reliably retract. These extra neurites even go on to form synapses in ectopic locations. Our goal here was to determine how Fat3, presumably through its asymmetric localization, regulates these fundamentally different cellular events (cell migration, neurite retraction, synapse formation) and confers polarity to the circuit.
We found that Fat3 binds intracellularly to several cytoskeletal effectors and synaptic proteins. By generating mouse strains in which Fat3 lacks specific binding sites for several different effectors, we showed that different combinations of motifs are required for directed migration of amacrine cells versus retraction of their neurites or the formation of properly localized synapses. Moreover, upon deletion of most of the intracellular domain, extra neurites form but do not make ectopic synapses. This surprising observation suggests that while neurites may form synapses opportunistically in some contexts, Fat3 can also directly influence synaptogenesis. In fact, removal of the intracellular domain also reduced formation of GABAergic synapses in the appropriate location. Thus, Fat3 not only determines whether cells and their processes are in the right location, but also where their synapses form.
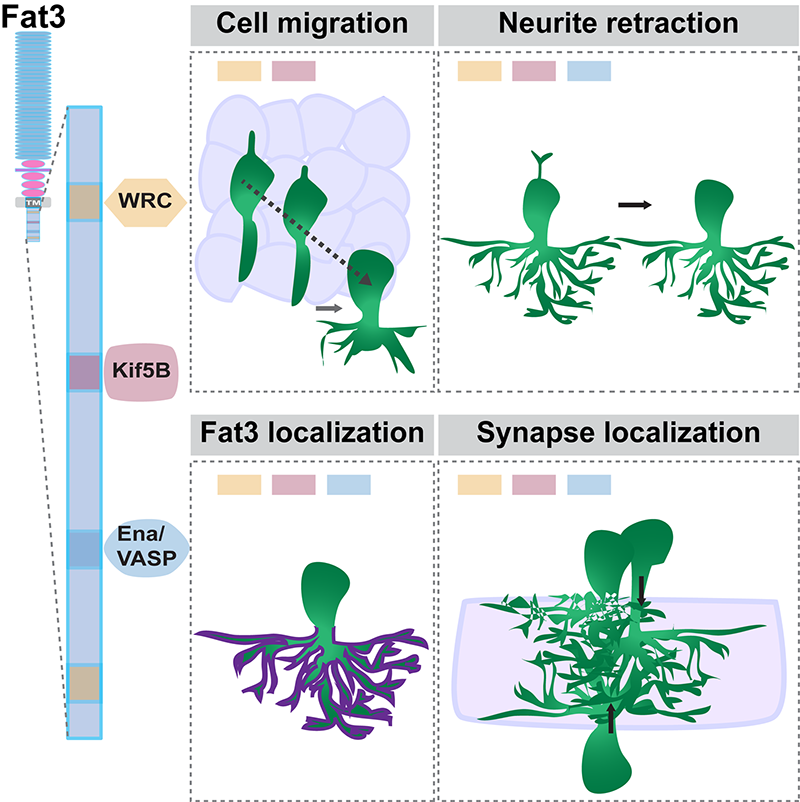
Graphical abstract of the study. On the left is a diagram of the Fat 3 molecule, showing three different “motifs” or protein binding sites within the intracellular domain. On the right the boxes show that Fat3 coordinates different aspects of neural circuit assembly by using different sets of intracellular effectors.
Taken together, our work suggests that Fat3 functions as a molecular node to coordinate multiple, independent polarization events, similar to the classic role of tissue polarity proteins in epithelial structures.
Evelyn Avilés is a postdoc in the laboratory of Lisa Goodrich in the Neurobiology Department at Harvard Medical School.
Learn more in the original research article:
Fat3 acts through independent cytoskeletal effectors to coordinate asymmetric cell behaviors during polarized circuit assembly.
Avilés EC, Krol A, Henle SJ, Burroughs-Garcia J, Deans MR, Goodrich LV. Cell Rep. 2022 Feb 1;38(5):110307.
News Types: Community Stories
