HOME / FOR EVERYONE / PHOTOS /
2019 Beauty of the Brain Image Contest
We are excited to share with you a gallery featuring submissions from our 2019 Beauty of the Brain contest, which sought to highlight spectacular research images obtained by students, postdocs and lab staff from all parts of the Harvard University and our affiliated hospitals. We hope these images spark curiosity and awe about the nervous system and might serve as an entry point for scientists and non-scientists alike to explore new research topics and methods. Six winning images were selected, and each winner received $200 and a customized desk plaque with their image.
Click on any image to see a larger version and then use the arrows to the left and right to go through the entire gallery at high resolution.
Interested in the images from our 2018 image contest? Please click here.
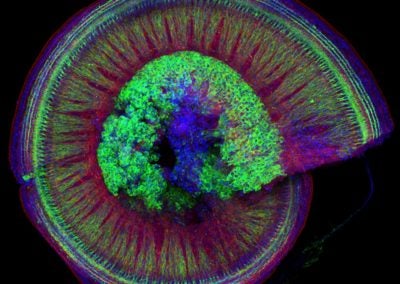
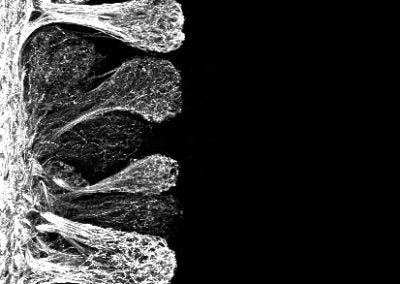
Wholemount of the mouse cochlea (Isle Bastille, Goodrich lab, HMS)
This spiraling organ residing in the mammalian inner ear is responsible for receiving sounds from the environment and transmitting them through neurons (green in the picture, cell bodies in the center) to the brain. The red is phalloidin which binds to the internal skeleton (actin) of the other cells in this organ (hair cells and support cells). The blue is a stain for DAPI, which binds to DNA in the nucleus of each cell.
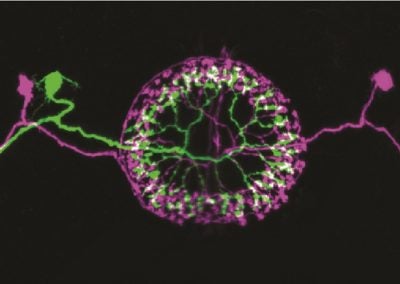
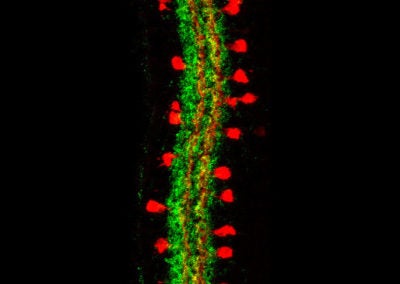
Multicolor FlpOut clones of Drosophila ellipsoid body ring neurons (Isabel D’Alessandro, Wilson lab, HMS)
Two ring neurons are labeled on opposite sides of the fruit fly brain. These neurons convey visual information to the brain’s internal ‘compass’ and are important for establishing and maintaining the fly’s sense of direction.
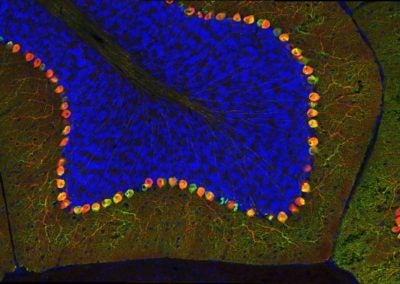
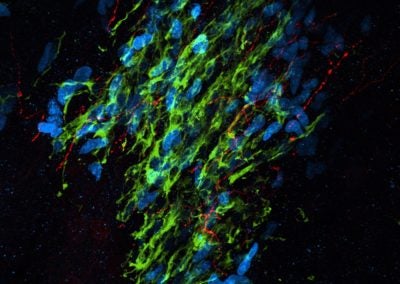
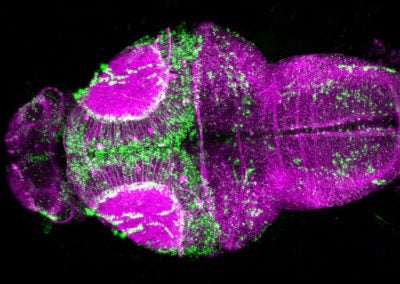
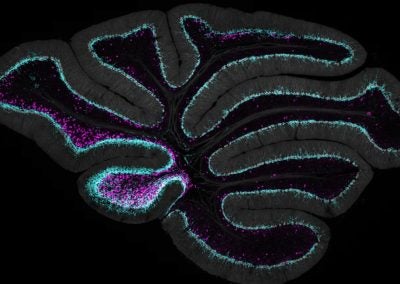
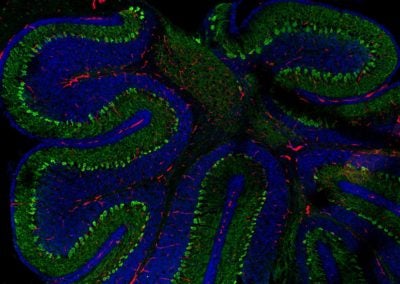
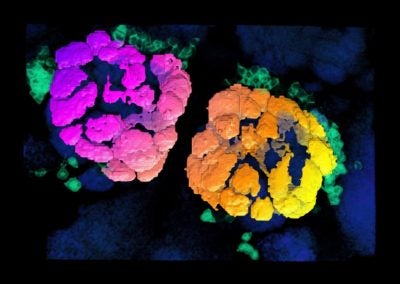
Cerebellar checkers (Ellen DeGennaro, Walsh lab, BCH)
This image shows a thin slice from an adult mouse cerebellum, highlighting blue nuclei, and Purkinje cells in red and green. Our lab is studying a gene that is expressed by some Purkinje cells but has different functions in different parts of the brain.
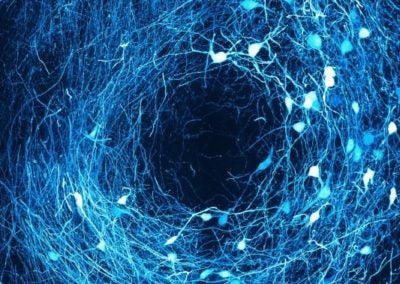
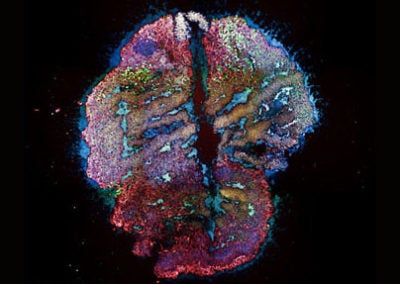
Ring in the brain (SNr) (Jaeeon Lee, Sabatini lab, HMS)
The image depicts cells inside SNr, a region of the brain responsible for initiating movement. Dendritic processes within SNr communicate each other and forms rings in the brain.
Olfactory bulb glomeruli (Joseph Zak, Murthy lab, HMS)
Axon terminals of olfactory sensory neurons terminating in the olfactory bulb at structures called glomeruli. All sensory neurons of a common type project to the same glomerulus and enter as a dense bundle of fibers. Axons are labeled with green fluorescent protein.
The starburst amacrine cells of the retina (Evelyn Aviles, Goodrich lab, HMS)
The image shows a type of neuron in the retina, the starburst amacrine cells (red), and synapses in the inner plexiform layer (green)
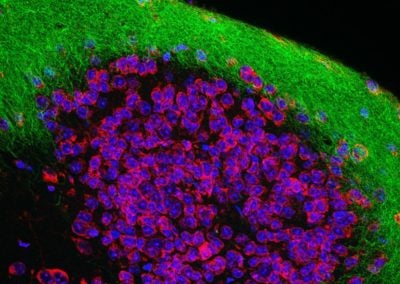
Serotonin fibers in the rostral migratory stream (Nikita Sturrock, Dymecki lab, HMS)
Serotonin neuron fibers (red) intertwining with proliferating neuroblasts labeled with Ki67 (blue), and doublecortin (green), migrating from an adult stem cell niche called the subventricular zone to their final destination in the olfactory bulb.
Man-made brain (Annie Kathuria, Karmacharya lab, MGH)
Human stem cell derived brain organoid slice stained with various neuronal markers.
Local interneurons in the drosophila antennal lobe (Isabel D’Alessandro, Wilson lab, HMS)
Several local interneurons are stochastically labeled in the antennal lobe, the primary olfactory center in the fruit fly brain. These local neurons play an important role in odor information processing.
The skin of a mouse ear (Brian W Chow, Gu lab, HMS)
Nerves, blood vessels and immune cells in the skin of a mouse ear
Developing motor neurons and muscles in E11.5 mouse (Jess Bell & Mary Whitman, Engle lab, BCH)
This image depicts a developing mouse embryo with muscles in red, and motor neurons in green.
Miami on the brain (Natasha M O’Brown, Gu and Megason labs, HMS)
Larval zebrafish brain expressing fluorescent protein throughout the entire brain and injected with a green tracer that leaks into the brain at this early stage.
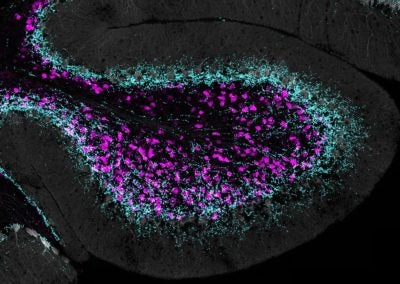
Unipolar brush cells contacted by Purkinje cell feedback (Chong Guo, Regehr lab, HMS)
Unipolar brush cells (in magenta) form a feed-forward network in specialized regions in the cerebellum. Coincidentally, in the same areas where they are found, there is an enrichment of Purkinje cell synapses, which allow the cerebellar output to feedback to the network of unipolar brush cells. These are example of novel circuit elements in the cerebellum which may serve a unique function in controlling balance and eye movement.
Unipolar brush cells contacted by Purkinje cell feedback (Chong Guo, Regehr lab, HMS)
Unipolar brush cells (in magenta) form a feed-forward network in specialized regions in the cerebellum. Coincidentally, in the same areas where they are found, there is an enrichment of Purkinje cell synapses, which allow the cerebellar output to feedback to the network of unipolar brush cells. These are example of novel circuit elements in the cerebellum which may serve a unique function in controlling balance and eye movement.
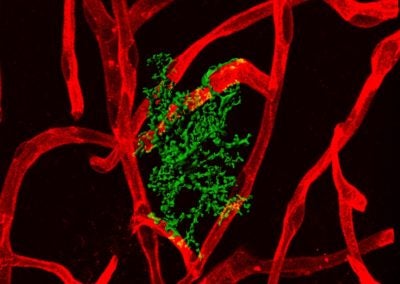
Astrocyte-blood vessel interaction (Urs Langen, Gu lab, HMS)
Astrocytes in contact with blood vessels in the mouse brain.
Astrocyte-blood vessel interaction (Urs Langen, Gu lab, HMS)
Astrocytes in contact with blood vessels in the mouse brain.
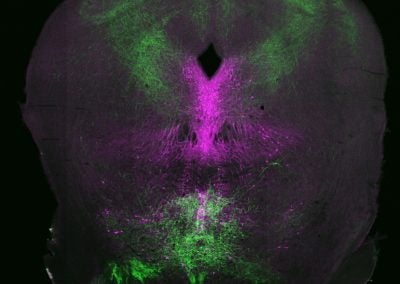
Rising from splashing waves in a thundery night (Giacomo Maddaloni, Dymecki lab, HMS)
In this coronal section of the adult mouse brain, shown in magenta are neurons that produce the neurotransmitter serotonin and in green are axons coming from neurons infected with a viral vector. Those axons surround serotonin neurons, just like splashing sea waves (bottom) and thunder (top) on a stormy night.
The brain in time (snake representation) (William Orwig, Sepulcre lab, MGH)
This image demonstrates dynamic changes in brain structure over time. Each cluster of nodes represents the functional connectivity of an individual brain at a given point. The alignment of each cluster, relative to those adjacent, indicates the stability of brain states over time.
The brain in time (flower representation) (William Orwig, Sepulcre lab, MGH)
This image demonstrates dynamic changes in brain structure over time. Each cluster of nodes represents the functional connectivity of an individual brain at a given point. The connectivity of each cluster to those adjacent and to the central configuration, indicate the stability of brain states over time.
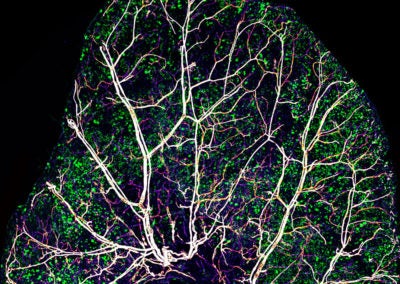
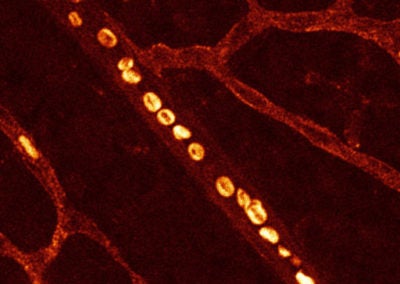
Red blood cells in retina vasculature (Swathi Ayloo, Gu lab, HMS)
This image is a snapshot of the rapidly moving red blood cells in an artery within the retina of a 10-day old mouse. The doughnut shape of these cells that enables them to effectively supply oxygen to tissues can be appreciated at this resolution.
Purkinje neurons in cerebellum (Swathi Ayloo, Gu lab, HMS)
This image captures the stereotypical arrangement of Purkinje neurons, shown in green, in the cerebellum of 10-day old mouse. Nuclei of the various cells in the cerebellum are shown in blue and in red are the blood vessels that supply oxygen and other nutrients to these cells.
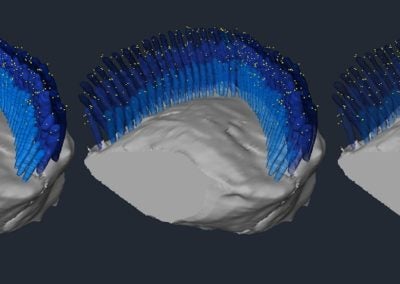
A 3D reconstruction of an anti-PKHD1L1 immunogold labeled OHC stereocilia bundle (Maryna Ivanchenko, Corey lab, HMS)
Outer hair cell stereocilia bundle labeled with anti-PKHD1L1 antibodies (10 nm gold), reconstructed from 387 serial FIB-SEM cross-sections, at 15 nm milling step. Each yellow dot represents a gold bead at the surface of three rows of hair cell stereocilia, shown in blue. The stereocilia are rendered with increasing level of transparency to highlight the gold beads between and behind the cilia.
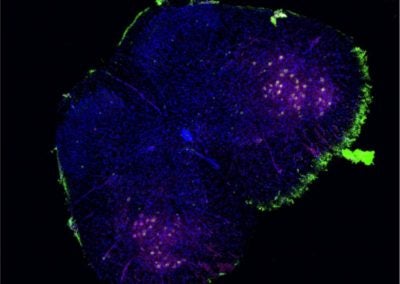
Projection neurons of the fruit fly antennal lobe (Matthew Churgin, de Bivort lab, Harvard)
The antennal lobe (AL) is the fruit fly’s smell center. Dendrites of the projection neurons (PNs) ramify throughout discrete clusters of the AL called glomeruli (purple->yellow coloring indicates depth). Each glomerulus relays signals from one of fifty classes of odor receptor. PN cell bodies (teal) reside just outside the AL.
Lateral olfactory tract (David Brann, Datta lab, HMS)
Olfaction is supported by a simple architecture: sensory neurons in the nose connect to higher-order olfactory regions in the brain with just two synapses. Using viral techniques to comprehensively label olfactory projections (green), we observe how they snake around and synapse with neurons in the olfactory tubercle (blue, red).
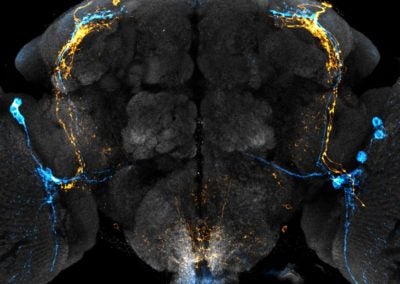
Clock neuron subpopulations in Drosophila melanogaster (Bryan Song, Rogulja lab, HMS)
Circadian rhythms control the timing of daily behaviors like sleep. Clock neurons in the brain coordinate rhythmic biological processes. In Drosophila melanogaster (aka fruit flies), the clock neuron network is composed of distinct subtypes. Two subtypes, in orange and blue, are shown within the fly brain (grey).
The olfactory mantle (David Brann, Datta lab, HMS)
A large portion of the ventral surface of the rodent brain is devoted to olfaction. Here, we visualized the axons from olfactory projection neurons in the olfactory bulb (in white), which send distributed projections to these ventral olfactory regions. <SA:Struct
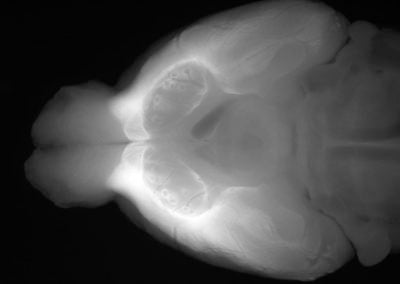
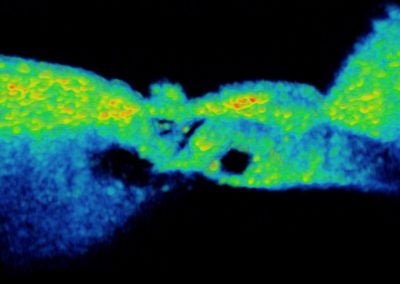
In vivo Organ of Corti (Nam Hyun Cho, Puria lab, MEEI)
The first 3d volume measurement of the intact organ of corti in a gerbil using optical coherence tomography.
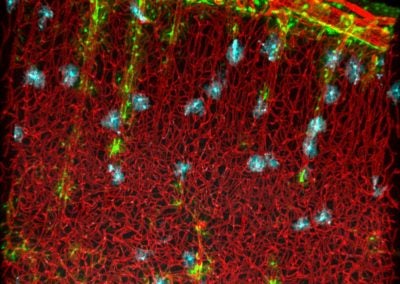
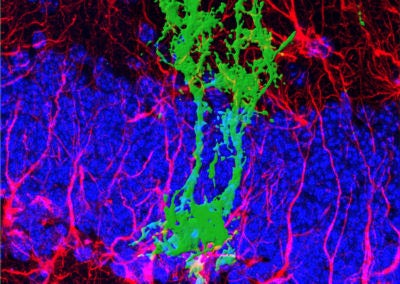
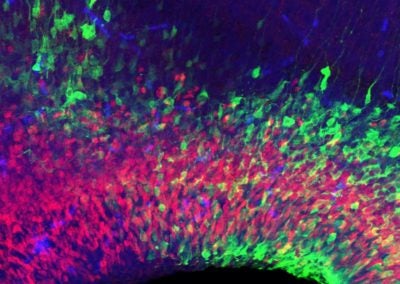
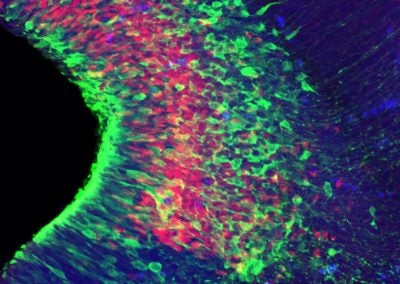
Symmetric division of a neural stem cell (Haley Zanga, Sahay lab, MGH)
This reconstructed image shows a genetically labeled neural stem cell (green) undergoing symmetric division in the dentate gyrus of a mouse. Astrocytes and radial glia cells stained in red. Nuclear marker DAPI is shown in blue.
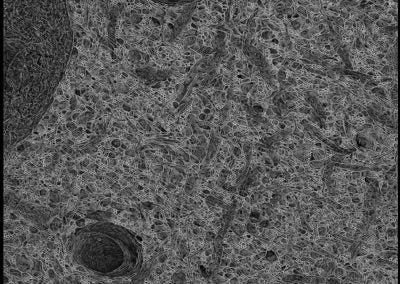
mEMbrain (Elisa Pavarino, Lichtman lab, Harvard)
The images are a projection of membrane predictions. The membranes were automatically predicted with deep learning tools on a volume of mouse cortex of the Lichtman Lab. Clearly distinguishable are two capillaries, a cell body and its nucleus, and a plethora of processes.
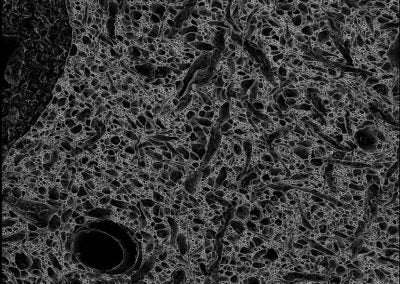
mEMbrain (Elisa Pavarino, Lichtman lab, Harvard)
The images are a projection of membrane predictions. The membranes were automatically predicted with deep learning tools on a volume of mouse cortex of the Lichtman Lab. Clearly distinguishable are two capillaries, a cell body and its nucleus, and a plethora of processes.
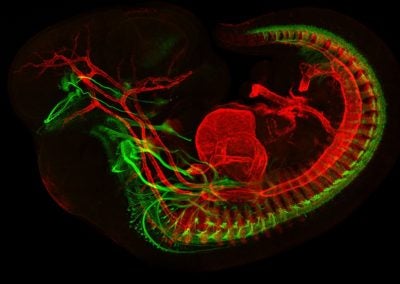
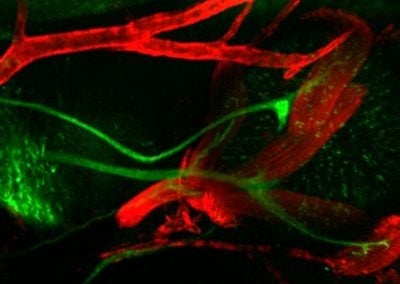
E11.5 orbit showcasing developing extraocular muscles and cranial nerves (Jess Bell & Mary Whitman, Engle lab, BCH)
This image shows extraocular muscles (red) and the cranial nerves (green) responsible for their control.
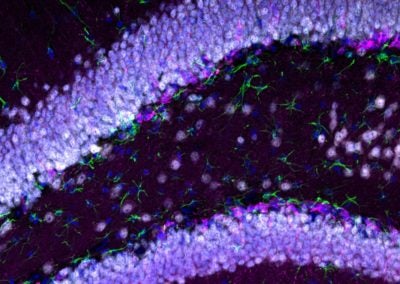
Birth of new neurons in the adult mouse brain (Brittany Mayweather, Rubin lab, Harvard)
Fluorescence microscope image depicting a region of the mouse brain responsible for memory formation. Blue labels individual cells and cell type identity coincides with the following additional color: white identifies mature neurons, pink identifies newborn neurons, and green labels a cell type essential for supporting neuronal functions.
Spinal motor neurons (Spencer Price, Greenberg lab, HMS)
Fluorescently labeled motor neurons in a mouse spinal cord section. Motor neurons are visualized through genetically-driven expression of a green fluorescent marker on the cell nucleus. The cell bodies of these motor neurons are revealed using a stain against ChAT, a marker enzyme of spinal motor neuron function.
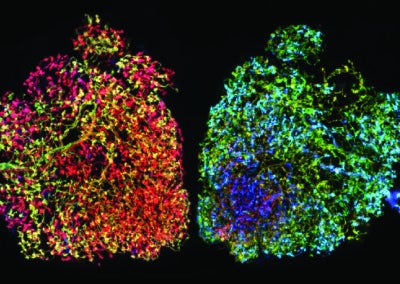
Ferret phases (Ellen DeGennaro, Walsh lab, BCH)
These images each encompass one half of a juvenile ferret brain, sliced into thin sections. One week after birth, the ferret brain is just developing its characteristic folds, similar to the familiar wrinkles of the human brain, and we can study how far cells travel across the brain during development.
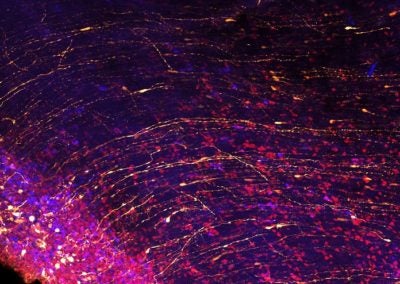
Cortical sparks (Lariza Rento, Walsh lab, BCH)
Neural progenitor cells show off their long apical fibers in the developing brain of a week old ferret.
Brain waves (Lariza Rento, Walsh lab, BCH)
The apical fibers of neural progenitor cells in a juvenile ferret brain sweep around the bend of a developing fold in the tissue.
Neuronal plumage (Katherine Morillo, Walsh lab, BCH)
Dividing neurons expressing different genes fan out in the developing mouse brain.